Abstract
Background
Fetal alcohol spectrum disorder (FASD) is a lifelong condition. Early interventions targeting core neurocognitive deficits have the potential to confer long-term neurodevelopmental benefits. Time-targeted choline supplementation is one such intervention that has been shown to provide neurodevelopmental benefits that emerge with age during childhood. We present a long-term follow-up study evaluating the neurodevelopmental effects of early choline supplementation in children with FASD approximately 7 years on average after an initial efficacy trial.
Methods
The initial study was a randomized, double-blind, placebo-controlled trial of choline vs. placebo in 2.5 to 5 year olds with FASD. Participants in this long-term follow-up study include 18 children (9 placebo; 9 choline) seen 7 years on average following initial trial completion. The mean age at follow-up was 11.0 years old. Diagnoses were 28% fetal alcohol syndrome (FAS), 28% partial FAS, and 44% alcohol-related neurodevelopmental disorder. The follow-up included measures of executive functioning and an MRI scan.
Results
Children who received choline had better performance on several tasks of lower-order executive function (e.g., processing speed) and showed higher white matter microstructure organization (i.e., greater axon coherence) in the splenium of the corpus callosum compared to the placebo group.
Conclusions
These preliminary findings, although exploratory at this stage, highlight potential long-term benefits of choline as a neurodevelopmental intervention for FASD and suggest that choline may affect white matter development, representing a potential target of choline in this population.
Trial registration
Prior to enrollment, this trial was registered with clinicaltrials.gov (NCT01149538) on June 23, 2010.
Similar content being viewed by others
Introduction
Fetal alcohol spectrum disorder (FASD) is an umbrella term describing a group of neurodevelopmental conditions resulting from prenatal alcohol exposure (PAE). The term FASD encompasses subtypes including fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (PFAS), and alcohol-related neurodevelopmental disorder (ARND). Importantly, while these conditions represent a spectrum of severity, research has demonstrated that neurodevelopmental anomalies and cognitive deficits occur across all of these subtypes, including in those without the characteristic facial features associated with PAE (i.e., those with ARND) [1, 2]. These conditions can include neurological impairment, cognitive and behavioral deficits, growth abnormalities, and facial dysmorphology [3, 4]. Global estimates place the prevalence of FASD at 0.8% [5, 6], and recent (and conservative) estimates place the prevalence at 1 to 5% in the USA [5]. Recent evidence indicates that rates of alcohol consumption are increasing globally [7, 8], suggesting a growing risk for alcohol-exposed pregnancies. Despite the high prevalence of FASD, this set of conditions is often unrecognized and misdiagnosed [9, 10], and few interventions have been developed to beneficially alter the lifelong neurodevelopmental course in this population [11, 12]. A large body of evidence has documented a wide range of neurocognitive deficits in FASD, which can include global intellectual deficits, as well as impairment in specific domains of attention, executive functioning, memory, visual-perceptual and motor skills, academic achievement, language skills, adaptive function, and social cognition [3, 13,14,15,16]. Even when global cognitive function (i.e., IQ) is not impaired, individuals with FASD can show marked impairment in specific cognitive skills such as executive function and memory [17].
Because of its role in regulating key aspects of brain development [18, 19], including hippocampal development, and its demonstrated capacity to attenuate neurodevelopmental damage from prenatal alcohol exposure in animal models [20,21,22], the essential nutrient choline has been studied as a potential intervention for the cognitive impairment associated with FASD [23,24,25]. Choline is known to have direct effects on brain development and function in both typical and atypical development [18]. Choline’s effects are believed to result from its impact on several neurodevelopmental mechanisms and processes. First, choline regulates DNA methylation and gene expression in the brain [26, 27]. It is also integral to the formation of phospholipids involved in cell membranes, axonal growth, and myelination [28, 29]. Choline also acts as a precursor to acetylcholine, which plays an important role in neurotransmission critical for memory and cognition [19, 30,31,32,33,34]. Preclinical studies using animal models of PAE have examined choline supplementation in the perinatal period and demonstrated beneficial effects on brain development [35], motor and behavioral development [36], memory [37, 38], and spatial learning [20]. Our series of studies examining the impact of choline supplementation on brain functions dependent on the hippocampus and on white matter represents a translation of preclinical models to the human. We tested the intervention only up to 5 years of age (when brain growth remains rapid) based on evidence from preclinical studies showing choline’s efficacy during an equivalent window [20]. Similarly, we chose sequential memory measures for our outcome based on preclinical evidence that choline has a direct impact on hippocampal development [39,40,41].
Few studies have examined choline supplementation in humans (for a comprehensive review, see [24, 27]). Several studies of prenatal (gestational) choline supplementation have suggested that choline supplementation mitigates PAE-related brain volume reductions and improves behavioral outcomes in infants with PAE [25, 42,43,44]. Research conducted to date suggests there may be a critical period of intervention in the prenatal stage and early childhood (i.e., birth to ages 3–4 years), with prenatal choline supplementation likely leading to larger effects on neurodevelopment in comparison with postnatal supplementation [27]. Our group at the University of Minnesota initially established the safety and tolerability of choline supplementation in children ages 2.5 to 5 years [45]. Our subsequent double-blind, randomized, placebo-controlled trial demonstrated age-related improvements on a developmentally sensitive measure of learning and memory in children ages 2.5 to 5 years who completed 9 months of daily choline compared to those in the placebo group [46]. Notably, we observed a steeper improvement in memory performance in younger children (ages 2.5 to 4 years) compared to older children (ages 4 to 5 years) in this sample. We conducted a 4-year follow-up study of children with PAE who participated in our initial clinical trial [23]. Larger and more consistent benefits for choline were observed at this later point in development. Participants in the choline group demonstrated higher nonverbal intelligence, visual-spatial skill, working memory ability, and verbal memory performance, as well as fewer parent-rated behavioral symptoms of ADHD compared to the placebo group. In contrast to these studies, a double-blind, placebo-controlled trial conducted by Nguyen and colleagues [47] found no group differences following a much shorter (6 weeks) choline supplementation in older children with PAE ages 5 to 10 years. Together, this research suggests there may be a critical period in early development for choline supplementation, with a protracted course of cognitive benefits that emerge over time. To our knowledge, potential long-term effects of early choline supplementation beyond 4 years have not been investigated, highlighting the need for further longitudinal research such as the study reported here.
In considering potential neurodevelopmental outcomes from choline supplementation, it is worth noting that the literature contains several decades of neuroimaging work highlighting a diverse range of neurological abnormalities associated with PAE, including reduced overall brain volume and regional gray matter volumes, differences in cortical thickness and gyrification, and abnormal gray and white matter longitudinal growth trajectories [48,49,50]. White matter (WM) abnormalities are among the most well-replicated neuroimaging findings in PAE and are thought to contribute to impaired functional connectivity and prominent deficits in attention and executive function in individuals with PAE [51,52,53,54,55]. Diffusion abnormalities in the corpus callosum (CC) are frequently observed in individuals with PAE and have been linked with other clinical features such as facial dysmorphology [56,57,58]. In typical development, the CC forms early in gestation and undergoes extensive morphological and volumetric change throughout childhood, with a period of rapid development during late childhood and adolescence [59, 60]. Frequent findings in PAE include reduced fractional anisotropy (FA), higher mean diffusivity (MD), and higher radial diffusivity (RD) compared to typically developing controls [54]. A number of studies have also observed shape abnormalities and overall volume reductions in the CC, particularly in posterior regions such as the splenium [54, 61, 62]. Such abnormalities in CC volume and microstructure have been associated with neurocognitive performance on tasks of eyeblink conditioning [63], working memory [55], mathematics [64], language and reading skill [65], and interhemispheric information transfer [66].
To date, a majority of studies examining white matter abnormalities in PAE have used traditional diffusion tensor imaging metrics such as FA and MD. Newer diffusion modeling techniques have the potential to provide greater specificity regarding WM microstructural abnormalities associated with neurodevelopmental conditions like FASD [54, 67]. For example, neurite orientation dispersion and density imaging (NODDI) [68] is a biophysical and multicomponent model that uses diffusion MRI data to characterize the density of neurites (i.e., axons and dendrites) as well as the alignment (dispersion) of tissue fibers within each voxel [69, 70]. The neurite density index (NDI) represents the intracellular volume fraction and is thought to reflect the density of axons (especially in white matter) and potentially dendrites (especially in gray matter). Neurite density is known to be functionally relevant, as evidenced by well-established relationships between dendritic arbor complexity and cognitive function [71]. In white matter, greater NDI values suggest axonal growth, greater axonal density, and/or myelination [70]. The orientation dispersion index (ODI) represents the angular variation of diffusion orientation and is thought to reflect the degree of bending and fanning of axons in white matter [68]. To our knowledge, no research has been conducted to date using NODDI to characterize WM abnormalities in PAE. NODDI has been used in several studies of neurodevelopmental conditions associated with WM microstructural abnormalities, such as prematurity [69, 72]. Given the role of choline in lipid synthesis, myelination, and axonal growth, as well as the greater specificity provided by NODDI metrics regarding tissue microstructure, this novel approach is well-suited for exploring potential microstructural WM differences associated with early choline supplementation in children and adolescents with PAE.
Here, we present data from a long-term (i.e., 7 years on average since initial trial) follow-up study of child participants treated with either choline or placebo in our initial randomized controlled trial of choline supplementation at ages 2.5 to 5 years of age. Examination of diffusion MRI and cognitive testing data from this long-term follow-up evaluation allowed us to explore potential lasting benefits of an early choline supplementation intervention targeting neurodevelopment. We examined domain-specific outcomes focusing on attention and executive functioning. Diffusion-weighted MRI and the NODDI biophysical model were used to explore potential differences in CC WM microstructure between treatment groups. Our reason for focusing on NODDI rather than additionally examining traditional diffusion metrics (e.g., FA, MD) was twofold: first, due to the small sample size, we aimed to limit the number of outcome measures examined; second, literature to date suggests NODDI provides a more biologically relevant method of modeling diffusion data due to its multi-compartment approach and better correlation with histology compared to traditional diffusion tensor modeling [69]. Therefore, in this study, we chose to implement the most advanced, most biologically relevant, and (theoretically) most sensitive diffusion imaging model. In this study, we specifically focused on the CC because of the large body of evidence highlighting the vulnerability of this region to PAE [54], as well as associations of CC diffusion anomalies to neurocognitive functioning in PAE [55, 63,64,65,66]. We also explored the relationship of CC microstructure to facial dysmorphology given previous findings in some studies of a relationship between CC anomalies (e.g., shape, thickness, diffusion) and facial features in PAE [56,57,58, 73, 74]. Given the limited sample size of this study, analyses were exploratory in nature.
Materials and methods
Parent-study methods and participants
Participants in the current long-term outcome study were children with PAE who took part in an earlier clinical trial of choline supplementation [46]. Detailed materials and methods for this earlier clinical trial and the 4-year follow-up study have been previously published [23, 45, 46] and will, therefore, be briefly summarized here. This was a randomized, double-blind, placebo-controlled study in which participants were randomized to receive either choline (1.25 g choline bitartrate powder mix delivering 513 mg choline) or matching placebo for 9 months (NCT01149538). A complete description of methods and procedures used in this clinical trial was reported in [45], and results of the initial trial were reported in [46].
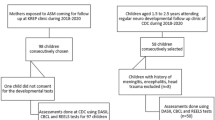
A total of 60 participants with FASD between the ages of 2.5 to 5 years were enrolled between June 2010 and May 2014 following a screening process to determine eligibility as well as IRB-approved consent process (Fig. 1). Initial exclusion criteria were the presence of another developmental disorder (e.g., autism, Down syndrome), low birth weight (< 1500 g), neurological disorder, traumatic brain injury, or other medical conditions affecting the brain. Participants were primarily recruited from the University of Minnesota Fetal Alcohol Spectrum Disorder Clinic and/or Adoption Medicine Clinic. Participants received the allocated intervention of choline or placebo (1:1 allocation to parallel groups), of which 85% (n = 51) completed the 9-month trial [46]. Investigators, staff, and participants were blinded to the treatment assignment. Parents administered the study drug to participants once daily for 9 months. Compliance was measured by calendar logs and packet counts with both groups averaging 88% days with a dose taken. In the initial follow-up, the Mullen Scales of Early Learning [75], which served as a primary outcome and a measure of global cognitive functioning, was administered at baseline and 9 months (study completion). An elicited imitation (EI) paradigm [76, 77] provided a measure of hippocampal-dependent sequential memory at baseline and 9 months. The Child Behavioral Checklist (CBCL) [78] was completed by parents at baseline and study completion.
Follow-up methods
Of those who completed the initial trial, 31 participants returned for a 4-year follow-up visit (15 choline and 16 placebo) between December 2015 and November 2017 to evaluate the longer-term neurodevelopmental effects of choline supplementation [23] (Fig. 1). New clinical diagnoses were recorded at the time of the 4-year follow-up visit. In the choline group, new diagnoses included 1 child with dyslexia and attention-deficit/hyperactivity disorder (ADHD). In the placebo group, new diagnoses included 1 child with autism spectrum disorder, 1 child with other specified depressive disorder and unspecified ADHD, and 1 child with anxiety. Because of the small number of diagnoses, we did not conduct any additional analyses on these data. A total of 17 participants (9 choline and 8 placebo) from the initial trial did not return for 4-year follow-up (lost contact or declined participation). Of the participants who returned, 4 (13%) were characterized as having FAS, 13 (42%) as having PFAS, and 14 (45%) as having alcohol-related neurodevelopmental disorder (ARND) based on the modified Institute of Medicine (IOM) criteria [79] with the height, weight, and head circumference measurements taken at initial enrollment [46]. Participants completed several cognitive measures assessing intellectual function (IQ), memory, attention, executive functioning, and inhibitory control. For more details about the 4-year follow-up, please refer to [23].
Subsequently, participants who were part of the 4-year follow-up study were asked to return for this long-term follow-up visit. The mean long-term follow-up duration was 7 years (range 4–10 years) after initial trial completion. A total of 18 participants (9 choline and 9 placebo; 5 females in each group) returned for neuroimaging and additional cognitive testing (Fig. 1). Participants were ages 8 to 15 years at long-term follow-up (mean age 11.0). A total of 6 choline and 7 placebo group participants did not return for this visit (lost contact, declined participation, or moved). Of the participants who returned, 5 (28%) had a diagnosis of FAS, 5 (28%) had a diagnosis of PFAS, and 8 (44%) had a diagnosis of ARND. Furthermore, within the choline group, 2 (22%) had FAS, 2 (22%) had pFAS, and 5 (57%) had ARND. In the placebo group, 3 (33%) had FAS, 3 had pFAS (33%), and 3 (33%) had ARND. All subtypes require some form of cognitive or behavioral impairment and a history of PAE. FAS also requires facial dysmorphology, growth deficiency, and a brain growth deficiency. In addition to cognitive or behavioral impairment, PFAS requires facial dysmorphology. ARND only requires a history of PAE and cognitive or behavioral impairment [4]. Research staff contacted participants via phone, email, and mailed letters. Participants were re-consented using IRB-approved consent and assent processes and forms. During a single visit, participants completed neurocognitive testing and MRI scanning. The last of these visits took place in 2021.
Given attrition of participants at the 4-year and 7-year follow-up studies, we compared participants who did and did not return in terms of overall cognitive function using independent-samples t-tests to test for potential selection bias in the returning participants. Participants who returned for the 4-year and 7-year follow-up studies did not differ from those who did not return regarding baseline cognitive function measured at the start of the original choline supplementation trial. Similarly, participants who did not return for the 7-year study did not differ in terms of cognitive function measured during the 4-year study.
Executive functioning
Participants completed the Digit Span and Picture Span subtests of the Wechsler Intelligence Scale for Children, 5th Edition (WISC-IV) [80] and the Trail-Making and Color-Word Interference subtests from the Delis-Kaplan Executive Functioning System (D-KEFS) [81]. These cognitive tests measure higher-level executive functioning skills (i.e., working memory, inhibitory control, switching/cognitive flexibility) as well as “lower-order” processes (e.g., visual scanning, attention, processing speed). Test scaled scores (i.e., mean of 10, standard deviation of 3) were used in all analyses.
MRI acquisition and processing
MRI data were acquired on a Siemens 3T Prisma scanner (Erlangen, Germany) equipped with a 32 channel head coil. For each participant, T1-weighted and T2-weighted scans were acquired using custom pulse sequences, which included automatic real-time motion detection and k-space line rejection and replacement software [82]. Pulse sequence parameters were chosen to match those used in the Lifespan Human Connectome Project in Development [83]. Selected parameters are described in Table 1.
All MRI data were initially preprocessed using the Human Connectome Project’s Minimal Preprocessing Pipeline (v4.0.1) [84]. For structural data, these steps included alignment of the T1-weighted volume to the T2-weighted volume and correction for gradient distortion and intensity bias before running FreeSurfer (v6.0.0), which performs subcortical segmentation, creates pial and white matter surface meshes, and neocortical parcellation [85, 86]. Diffusion preprocessing included rigid AC-PC alignment to the structural images, correction for susceptibility-related distortions using FSL’s top-up, and eddy current distortion correction and slice outlier replacement using FSL’s eddy tool (v 6.0.1).
Tractography to delineate major white matter tracts was carried out using the TRActs Constrained by Underlying Anatomy (TRACULA) tool available in the FreeSurfer v7.2.0 release [87]. TRACULA identifies 42 major white matter tracts based on probabilistic tractography informed by expertly defined anatomical priors. Voxel-wise NODDI metrics were estimated using the Accelerated Microstructure Imaging via Convex Optimization (AMICO) toolkit [88]. Finally, for each metric and tract of interest, tract-wise averages were computed, weighting each voxel based on likelihood of tract membership and excluding any voxels with less than 20% of the robust maximum probability of tract membership. For this analysis, we focused on the eight major callosal tracts defined by TRACULA (Fig. 2): the central, parietal, premotor, prefrontal, and temporal projections from the callosal body and the genu, rostrum, and splenium. An experienced rater (DJR) inspected all raw images, FreeSurfer segmentations and surface parcellations, tractography results, and NODDI scalar maps. The rater determined that no data should be excluded based on quality or aberrant processing.
Statistical analyses
Statistical analyses were performed with R version 4.1.1 [89]. First, independent sample t-tests and chi-square tests were used to examine treatment group differences (choline vs. placebo) in demographic variables and whole brain/hemispheric volume at follow-up visit. Initial data exploration (e.g., visual inspection of histograms) and Shapiro-Wilk tests revealed several outcome variables to be non-normally distributed (i.e., corpus callosum ODI values in the genu, premotor, body [central], body [parietal], and body [temporal]; D-KEFS Trail-Making Test visual scanning, number sequencing, letter sequencing, motor speed; D-KEFS Color-Word Interference Test color naming). As such, Mann-Whitney U-tests were used to compare treatment groups on mean NODDI metrics (NDI and ODI) in defined parts of the corpus callosum and cognitive outcomes at follow-up visit. Cohen’s d effect sizes were calculated and interpreted as small (d = 0.2), moderate (d = 0.5), or large (d = 0.8; [90]). Given known microstructural abnormalities of the corpus callosum associated with PAE, Kendall’s tau correlations were used to determine the relationship between NODDI metrics (in tracts with significant group differences in microstructure) and executive function test performance at follow-up testing (conducted at the same time as the follow-up MRI scan) at the whole-group level. Significance values were reported with and without correction for multiple comparisons given the number of correlations performed (11 in total) and the exploratory nature of these analyses. Significance values were corrected for multiple comparisons using the Holm-Bonferroni procedure [91, 92]. Lastly, exploratory analyses were performed to examine the relationship of facial dysmorphology to corpus callosum microstructure using both Kendall’s tau correlations (with number of dysmorphic facial features as a continuous variable [0 to 3 features]) and Mann-Whitney U-tests (with facial dysmorphology as a dichotomous variable [dysmorphic face = 2 or more facial features]).
Results
Participant characteristics
As shown in Table 2, the choline and placebo groups did not differ significantly on demographic variables including age at enrollment and age at follow-up evaluation, sex, ethnicity, and handedness (there were two participants for whom handedness data were not available). The groups also did not differ significantly with regard to total intracranial volume, overall intelligence, or physical characteristics (i.e., growth deficiency, microcephaly, and dysmorphic facial features). Similar to other studies of children with PAE, mean intelligence at 4-year follow-up (as measured by the Stanford-Binet Intelligence Scales, Fifth Edition; [93]) was significantly lower than the mean of the test normative sample (i.e., a mean IQ of 100). That is, the choline group mean IQ at 4-year follow-up (standard score of 90.5) was significantly lower than the test normative sample, t(7) = −2.63, p = 0.01, as was the placebo group mean IQ at 4-year follow-up (83.56), t(8) = −2.28, p = 0.02.
Despite randomization, there were significant group differences in racial identity between the choline and placebo groups. In general, participants in the placebo group who returned for follow-up evaluation were more racially diverse than those in the choline group. The three racial comparisons that were statistically significant included the proportion of American Indian/Alaska Native to White individuals (χ2 (1) = 5.63, p = 0.02), Black/African American to White (χ2 (1) = 4.44, p = 0.04), and White to non-White (χ2 (1) = 4.00, p = 0.04).
Executive functioning
Participants in the choline group demonstrated better mean performance than those in the placebo group across the majority of executive function measures at follow-up testing (Table 3). There were no significant group differences for the WISC-V working memory subtests (Digit Span and Picture Span) or the D-KEFS Color-Word Interference Test. Participants in the choline group demonstrated significantly higher mean performance than those in the placebo group on the D-KEFS Trail Making Trial of motor speed (Cohen’s d = 1.14; 18.2% difference) measuring the speed of visual-motor processing, representing a large effect size. For the D-KEFS Trail Making Color-Word Interference Test color naming trial, there was a trend (p = 0.05) toward higher performance in the choline group compared to the placebo group, representing a large effect size (d = 1. 27) and a 63.2% difference. Although the Color-Word Interference Test trials of inhibition and inhibition/switching were not statistically significant, the choline group demonstrated numerically higher performance than the placebo group. Effect sizes were moderate for group differences in inhibition (d = 0.72; 41.6% difference) and inhibition/switching (d = 0.78; 34.1% difference) performance.
White matter microstructure
Mann-Whitney U-tests were used to examine treatment group differences (placebo vs choline) for NODDI measures in the corpus callosum (Table 4). The choline group demonstrated significantly lower ODI (more coherent fibers with less bending/fanning) in the splenium of the corpus callosum compared to the placebo group, which represented a large effect (d = −1.26; 20.0% difference). No other significant treatment group differences were found in corpus callosum ODI. Although not statistically significant, the choline group showed marginally lower ODI in the temporal region of the body of the corpus callosum compared to placebo (6.9% difference), representing a moderate effect (d = −0.63). For NDI, there were no treatment group differences in the corpus callosum.
Relationship of cognitive performance to white matter microstructure
Correlation analyses were performed with the whole sample (i.e., collapsing diagnostic groups) to explore the relation of cognitive performance to white matter microstructure in the splenium of the corpus callosum because this region showed a significant difference between choline and placebo groups. Correlations with Kendall’s tau indicated performance on two executive function measures were negatively correlated with splenium ODI (Fig. 3): Digit Span scaled score (τb = −0.43, p = 0.03) and D-KEFS Word Reading scaled score (τb = −0.45, p = 0.03). Correction of significance values for multiple comparisons using the Bonferroni-Holm procedure revealed that neither correlation survived correction, possibly due to the small sample size and limited statistical power. Importantly, higher scores on these executive function measures indicate better performance, whereas lower ODI values represent less bending and fanning of axons (i.e., more coherent fibers). As such, the negative correlations observed here are in the expected direction: better executive function performance was associated with lower ODI values in the splenium. No other significant correlations were found.
Relationship of facial dysmorphology to corpus callosum white matter microstructure
The relationship between ODI in the splenium of the corpus callosum and facial dysmorphology (evaluated at baseline assessment prior to commencing choline supplementation) was also examined (Fig. 4). Results of a Kendall’s tau correlation between the total number of dysmorphic facial features (i.e., 0 to 3) and splenium ODI revealed a significant positive correlation in the whole sample (τb = 0.45, p = 0.02); a higher number of dysmorphic facial features was associated with lower microstructural organization. Similarly, a Mann-Whitney U-test with facial dysmorphology used as a dichotomous variable revealed significant differences between groups in splenium ODI. Participants without a clinically dysmorphic face (i.e., fewer than two facial features; median = 0.09) showed lower splenium ODI (better organization) compared to those with a clinically dysmorphic face (i.e., two or more facial features; median = 0.11, W = 12.0, p = 0.01).
Discussion
Data presented here from our longitudinal RCT follow-up study of children with FASD suggest potential long-lasting benefits to executive function skills approximately 7 years on average following early choline supplementation. These 7-year outcome data are consistent with our 4-year outcome data, which demonstrated benefits in nonverbal IQ, visual-spatial skills, working memory, verbal memory, and ADHD-related behavioral problems [23], and these effects extend to an age where the changes likely reflect stable long-term advantages. In a continuing trend first reported at the 4-year follow-up, the 7-year effect sizes were also larger and more consistent across measures than those initially observed immediately following trial completion [46]. Notably, effect sizes for several measures of executive function were moderate to large in magnitude in comparison with the moderate effect sizes (and nonsignificant group differences) observed at the 4-year point on similar tests of executive function [23]. Early preclinical investigations suggested a regionally specific effect of choline on hippocampal structure and function [94, 95], which was further supported by results from our first clinical trial in young children demonstrating an effect for postnatal choline supplementation on a hippocampal-dependent memory task but not overall cognitive function [46]. Indeed, similar studies investigating nutritional supplementation such as long-chain polyunsaturated fatty acid early in life suggest a “scaffolding” effect in which effects of intervention on early-developing foundational processes support later development of brain structures and functions (e.g., IQ, executive function) [96]. As such, one would expect long-term effects to be in a wider domain than the original effects of an early intervention [97], which aligns with findings of the current study of wider gaps in executive function performance at 7-year follow-up between choline and placebo participants compared to 4-year follow-up. Such treatment group differences may continue to widen with age, highlighting the need for further longitudinal research with longer-term follow-up evaluations.
In this 7-year follow-up sample, participants in the choline group had higher mean scores across a majority of executive function tests compared to those in the placebo group. A significant group differences (choline > placebo) was observed on the motor speed trial of the D-KEFS Trail Making Test, and a trend-level group difference (choline > placebo) was observed on the color naming trial of the D-KEFS Color-Word Interference Test. These results highlight the continued neurodevelopmental benefits of early choline supplementation in children with FASD. Our findings also suggest that benefits to aspects of executive functioning performance from early supplementation may become increasingly apparent in middle childhood and adolescence, which aligns with previous work suggesting there may be an early developmental window for choline’s therapeutic effects, and that such effects may emerge and increase across time [24, 47]. Future research should continue to investigate the neurocognitive benefits of early choline supplementation, including variations in dose, duration, and timing of choline in early childhood, as well as potential neurocognitive and behavioral benefits continuing into late adolescence and adulthood. An ongoing study by our group at the University of Minnesota (NCT05108974) is investigating several of these important questions, including evaluation of a weight-adjusted dose and random assignment of children with PAE ages 2.5–5 years to receive either 3 months of daily choline plus 6 months of placebo or 6 months of daily choline plus 3 months of placebo.
Our finding of a choline benefit for measurable aspects of executive function (i.e., processing speed) at 7 years post-intervention is encouraging and could plausibly translate to real functional benefits for children from early choline supplementation. Executive function skills develop along a nonlinear trajectory during childhood and adolescence [98,99,100] and parallel the maturation of the cerebral cortex and white matter [101,102,103]. In addition, executive function in childhood and adolescence is associated with long-term outcomes across the lifespan including social-emotional functioning, academic achievement, and risk for psychopathology [104]. Impairments in attention and executive function are hallmark features of FASD and have been repeatedly demonstrated in several studies [52, 105,106,107]. As such, these are meaningful targets for intervention in FASD, and our findings suggest that early choline supplementation may play an important role in conferring neurodevelopmental benefits for this uniquely vulnerable skillset.
Performance advantages for the choline group on the color-naming trial of the D-KEFS Color-Word Interference Test and the motor speed trial of the D-KEFS Trail Making Test may be consistent with the potential underlying white matter change observed here following choline supplementation. These tasks may reflect “lower-order” processes (e.g., visual scanning, attention to the task, speed of information processing, visuo-motor speed) that are necessary but not sufficient for performance on higher-level EF skills, such as inhibitory control and shifting/cognitive flexibility [108]. Age-corrected scaled scores are generated from the task completion time in seconds, and as such, these tasks provide a metric for lower-order skills such as speed of visuomotor processing and verbal output [109]. Consistent with our findings, past studies have documented an association between microstructural abnormalities in the posterior CC and cognitive performance on tasks measuring working memory, visual-motor integration, memory, and IQ [55, 110, 111]. Future studies with more than one neuroimaging time point could further examine relationships between choline supplementation and cognitive outcome via underlying brain mechanisms including white matter microstructure.
Unique to this study is the examination of diffusion MRI data using a biophysical model (i.e., NODDI) that allows for greater specificity with regard to white matter microstructure compared to traditional diffusion tensor imaging metrics such as FA and MD [54, 67]. We demonstrate white matter microstructural differences between treatment groups, suggesting early choline supplementation may affect white matter development. Specifically, we found significant group differences in white matter microstructure in the splenium of the corpus callosum (i.e., lower ODI). We also observed that, collapsing across groups, higher ODI values were associated with a greater number of dysmorphic features, consistent with several previous studies showing an association between corpus callosum integrity (volume and microstructure) and facial dysmorphology in PAE samples [56,57,58]. Abnormalities in the corpus callosum (genu, body, isthmus, and splenium) have been repeatedly demonstrated in PAE samples using traditional diffusion MRI metrics [54]. Specific findings have included reductions in FA and/or increases in MD, RD, or AD in these regions of the corpus callosum [55, 61, 65, 110, 112]. Here, we show that abnormalities in the corpus callosum (specifically the splenium) may be partially ameliorated by early choline supplementation. It is noteworthy that we did not find differences in NDI given the role of choline in lipid synthesis and myelination. Age-related increases in NDI in WM are thought to reflect myelination, axonal growth, or increases in axon density, and WM NDI tends to align better with FA than ODI [70]. In contrast, ODI changes little across child and adolescent development and provides a measure of neurite dispersion that points to coherence of axons and geometry [70]. Our findings of lower ODI in the splenium in the choline group could possibly reflect a beneficial developmental effect of early choline supplementation in terms of the structure and organization of axons in the corpus callosum. However, given that NODDI has been used in a limited number of studies with pediatric samples [69] and to our knowledge has not yet been used in studies of individuals with PAE, interpretation of our results remains provisional at this point. Together, our findings of treatment group differences in both brain microstructure and related cognitive function (e.g., processing speed) suggest a biologically plausible long-term effect of early choline supplementation in PAE.
Several limitations should be considered in interpreting results of the current study and informing future investigations. First, as a result of attrition, a limited number of initial choline supplementation trial participants returned for follow-up evaluation. Nonetheless, the returning groups were well matched with each other. Importantly, despite randomization, there were differences in racial identity between treatment groups. The size of our sample and limitations in statistical power meant that we were unable to explore potential confounding effects of group differences in racial identity on neuroimaging and neurocognitive outcomes, highlighting an important consideration for future research. Although the small sample size available for analysis at long-term follow-up limits the generalizability of our findings, it is noteworthy that significant treatment group differences and biologically plausible neuroimaging results were nevertheless identified in this small sample. A second limitation is the range of duration (ranging from 4 to 10 years) between trial completion and long-term evaluation in our sample. This is relevant because brain development and cognitive performance changes occur rapidly throughout childhood and into adolescence [98,99,100,101,102,103]. Age-corrected cognitive standard scores were used, and there was not a significant difference in the number of years to follow-up between the choline and placebo groups. Future choline studies could take into account age at MRI scanning and number of years since treatment. As recently illustrated in a comprehensive review, further longitudinal investigation into neurodevelopmental trajectories in PAE is needed [50]. A third limitation is the risk for inflated type 1 errors. Given the small sample size and exploratory nature of the current study, we chose to present the results without correction so as to avoid inflating the type 2 error rate (potentially missing important associations at this preliminary stage of the work) [113]. We carefully described patterns in our findings instead of focusing on isolated findings. Specifically, we found cognitive differences between the choline and placebo groups (e.g., processing speed) that were consistent across different tests, and our neuroimaging findings were consistent with previous research on white matter microstructure in children with PAE. Additional studies will be needed to replicate these findings.
Conclusions
Results of the current 7-year follow-up study suggest continued neurodevelopmental benefits of early choline supplementation in children with FASD that are detectable into middle childhood and adolescence. The MRI results suggest that the cognitive benefits are associated with white matter microstructural effects of choline supplementation. To our knowledge, this study is the first to report on NODDI in a sample of children with FASD.
Ultimately, children with FASD are likely to benefit from a combination of supports and interventions, and our results provide further evidence that early nutritional supplementation may play a role in supporting positive long-term developmental outcomes. Results presented here highlight the importance of continuing to assess for treatment effects of early neurodevelopmental interventions that may manifest over the course of development. Ongoing efforts at the level of public health and legislation will also continue to be crucial in supporting individuals affected by FASD, including improving diagnostic capacity and early detection, supporting prenatal care and addiction treatment, and further developing behavioral and other interventions that can meaningfully benefit children with FASD across the lifespan [1, 3, 11, 114].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Outcome data from the parent trial is available on ClinicalTrials.Gov.
Abbreviations
- ARND:
-
Alcohol-related neurodevelopmental disorder
- FAS:
-
Fetal alcohol syndrome
- FASD:
-
Fetal alcohol spectrum disorders
- CC:
-
Corpus callosum
- D-KEFS:
-
Delis-Kaplan Executive Functioning System
- NDI:
-
Neurite density index
- NODDI:
-
Neurite orientation dispersion and density imaging
- ODI:
-
Orientation dispersion index
- pFAS:
-
Partial fetal alcohol syndrome
- PAE:
-
Prenatal alcohol exposure
- WISC-V:
-
Wechsler Intelligence Scale for Children, 5th Edition
- WM:
-
White matter
References
Charness ME. Fetal alcohol spectrum disorders: awareness to insight in just 50 years. Alcohol Res. 2022;42:05. https://doi.org/10.35946/arcr.v42.1.05.
Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–21.
Wozniak JR, Riley EP, Charness ME. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 2019;18:760–70. https://doi.org/10.1016/S1474-4422(19)30150-4.
Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, et al. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics. 2016:138. https://doi.org/10.1542/peds.2015-4256.
May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, et al. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA. 2018;319:474–82. https://doi.org/10.1001/jama.2017.21896.
Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S. Global prevalence of fetal alcohol spectrum disorder among children and youth: a systematic review and meta-analysis. Obstet Gynecol Surv. 2018:189–91. https://doi.org/10.1097/01.ogx.0000532194.88210.00.
Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493–502. https://doi.org/10.1016/S0140-6736(18)32744-2.
Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37–42. https://doi.org/10.1016/j.alcohol.2021.06.004.
Chasnoff IJ, Wells AM, King L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics. 2015;135:264–70. https://doi.org/10.1542/peds.2014-2171.
McLennan JD. Misattributions and potential consequences: the case of child mental health problems and fetal alcohol spectrum disorders. Can J Psychiatry. 2015;60:587–90. https://doi.org/10.1177/070674371506001210.
Flannigan K, Coons-Harding KD, Anderson T, Wolfson L, Campbell A, Mela M, et al. A systematic review of interventions to improve mental health and substance use outcomes for individuals with prenatal alcohol exposure and fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2020;44:2401–30. https://doi.org/10.1111/acer.14490.
Petrenko CLM, Davis AS. Neuropsychological aspects of prevention and intervention for FASD: international perspectives. J Pediatr Neuropsychol. 2017;3:1–6. https://doi.org/10.1007/s40817-017-0036-1.
Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, et al. Neurobehavioral deficits consistent across age and sex in youth with prenatal alcohol exposure. Alcohol Clin Exp Res. 2016;40:1971–81. https://doi.org/10.1111/acer.13153.
Doney R, Lucas BR, Jones T, Howat P, Sauer K, Elliott EJ. Fine motor skills in children with prenatal alcohol exposure or fetal alcohol spectrum disorder. J Dev Behav Pediatr. 2014;35:598–609. https://doi.org/10.1097/DBP.0000000000000107.
Kodituwakku PW. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:218–24. https://doi.org/10.1002/ddrr.73.
Thorne JC. Accentuate the negative: grammatical errors during narrative production as a clinical marker of central nervous system abnormality in school-aged children with fetal alcohol spectrum disorders. J Speech Lang Hear Res. 2017:3523–37. https://doi.org/10.1044/2017_jslhr-l-17-0128.
Eme R, Millard E. Fetal alcohol spectrum disorders: a literature review with screening recommendations. Display Ad Rates. 2012;13 Available: http://apadivision16.org/wp-content/uploads/2015/12/TSP-Vol.-66-No.-1-January-2012.pdf#page=12.
Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. https://doi.org/10.1111/j.1753-4887.2006.tb00202.x.
Zeisel SH. The fetal origins of memory: the role of dietary choline in optimal brain development. J Pediatr. 2006;149:S131–6. https://doi.org/10.1016/j.jpeds.2006.06.065.
Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. https://doi.org/10.1016/j.brainres.2008.08.048.
Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–11. https://doi.org/10.1016/S0892-0362(00)00097-0.
Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–30.
Wozniak JR, Fink BA, Fuglestad AJ, Eckerle JK, Boys CJ, Sandness KE, et al. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J Neurodev Disord. 2020;12:9. https://doi.org/10.1186/s11689-020-09312-7.
Ernst AM, Gimbel BA, de Water E, Eckerle JK, Radke JP, Georgieff MK, et al. Prenatal and postnatal choline supplementation in fetal alcohol spectrum disorder. Nutrients. 2022;14. https://doi.org/10.3390/nu14030688.
Jacobson SW, Colin Carter R, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, et al. Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2018:1327–41. https://doi.org/10.1111/acer.13769.
Sarkar DK, Gangisetty O, Wozniak JR, Eckerle JK, Georgieff MK, Foroud TM, et al. Persistent changes in stress-regulatory genes in pregnant women or children exposed prenatally to alcohol. Alcohol Clin Exp Res. 2019;43:1887–97. https://doi.org/10.1111/acer.14148.
Akison LK, Kuo J, Reid N, Boyd RN, Moritz KM. Effect of choline supplementation on neurological, cognitive, and behavioral outcomes in offspring arising from alcohol exposure during development: a quantitative systematic review of clinical and preclinical studies. Alcoholism Clin Exp Res. 2018:1591–611. https://doi.org/10.1111/acer.13817.
Bekdash RA. Neuroprotective effects of choline and other methyl donors. Nutrients. 2019:2995. https://doi.org/10.3390/nu11122995.
Zeisel SH. The supply of choline is important for fetal progenitor cells. Semin Cell Dev Biol. 2011;22:624–8. https://doi.org/10.1016/j.semcdb.2011.06.002.
Magil SG, Zeisel SH, Wurtman RJ. Effects of ingesting soy or egg lecithins on serum choline, brain choline and brain acetylcholine. J Nutr. 1981;111:166–70. https://doi.org/10.1093/jn/111.1.166.
Conlay LA, Zeisel SH. Neurotransmitter precursors and brain function. Neurosurgery. 1982:524–9. https://doi.org/10.1097/00006123-198204000-00020.
Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988:339–53. https://doi.org/10.1002/dev.420210405.
Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997;8:2831–5. https://doi.org/10.1097/00001756-199709080-00005.
Cheng R-K, MacDonald CJ, Williams CL, Meck WH. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn Mem. 2008:153–62. https://doi.org/10.1101/lm.729408.
Sawant OB, Birch SM, Goodlett CR, Cudd TA, Washburn SE. Maternal choline supplementation mitigates alcohol-induced fetal cranio-facial abnormalities detected using an ultrasonographic examination in a sheep model. Alcohol. 2019;81:31–8. https://doi.org/10.1016/j.alcohol.2019.05.001.
Idrus NM, Breit KR, Thomas JD. Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 2017;59:43–52. https://doi.org/10.1016/j.ntt.2016.11.007.
Schneider RD, Thomas JD. Adolescent choline supplementation attenuates working memory deficits in rats exposed to alcohol during the third trimester equivalent. Alcohol Clin Exp Res. 2016;40:897–905. https://doi.org/10.1111/acer.13021.
Waddell J, Mooney SM. Choline and working memory training improve cognitive deficits caused by prenatal exposure to ethanol. Nutrients. 2017;9. https://doi.org/10.3390/nu9101080.
Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–7. https://doi.org/10.1037/0735-7044.120.2.482.
Otero NKH, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin Exp Res. 2012;36:1701–9. https://doi.org/10.1111/j.1530-0277.2012.01784.x.
Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999;113:13–20. https://doi.org/10.1016/S0165-3806(98)00183-7.
Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, et al. Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Matern Child Health J. 2015;19:2605–14. https://doi.org/10.1007/s10995-015-1779-x.
Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, et al. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol. 2015;49:647–56. https://doi.org/10.1016/j.alcohol.2015.08.005.
Warton FL, Molteno CD, Warton CMR, Wintermark P, Lindinger NM, Dodge NC, et al. Maternal choline supplementation mitigates alcohol exposure effects on neonatal brain volumes. Alcohol Clin Exp Res. 2021;45:1762–74. https://doi.org/10.1111/acer.14672.
Wozniak JR, Fuglestad AJ, Eckerle JK, Kroupina MG, Miller NC, Boys CJ, et al. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr Res. 2013:897–904. https://doi.org/10.1016/j.nutres.2013.08.005.
Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, et al. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2015:1113–25. https://doi.org/10.3945/ajcn.114.099168.
Nguyen TT, Risbud RD, Mattson SN, Chambers CD, Thomas JD. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am J Clin Nutr. 2016;104:1683–92. https://doi.org/10.3945/ajcn.116.142075.
Moore EM, Migliorini R, Infante MA, Riley EP. Fetal alcohol spectrum disorders: recent neuroimaging findings. Curr Dev Disord Rep. 2014;1:161–72. https://doi.org/10.1007/s40474-014-0020-8.
Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21:102–18. https://doi.org/10.1007/s11065-011-9163-0.
Moore EM, Xia Y. Neurodevelopmental trajectories following prenatal alcohol exposure. Front Hum Neurosci. 2021;15:695855. https://doi.org/10.3389/fnhum.2021.695855.
Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Hoecker HL, Boys CJ, et al. Global functional connectivity abnormalities in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:748–56. https://doi.org/10.1111/acer.12024.
Ware AL, Long X, Lebel C. Functional connectivity of the attention networks is altered and relates to neuropsychological outcomes in children with prenatal alcohol exposure. Dev Cogn Neurosci. 2021;48:100951. https://doi.org/10.1016/j.dcn.2021.100951.
Gautam P, Nuñez SC, Narr KL, Kan EC, Sowell ER. Effects of prenatal alcohol exposure on the development of white matter volume and change in executive function. Neuroimage Clin. 2014;5:19–27. https://doi.org/10.1016/j.nicl.2014.05.010.
Sherbaf FG, Aarabi MH, Yazdi MH, Haghshomar M. White matter microstructure in fetal alcohol spectrum disorders: a systematic review of diffusion tensor imaging studies. Hum Brain Mapp. 2019:1017–36. https://doi.org/10.1002/hbm.24409.
Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, et al. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res. 2009;33:1825–35. https://doi.org/10.1111/j.1530-0277.2009.01021.x.
Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, et al. Characterization of white matter microstructure in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:514–21. https://doi.org/10.1111/j.1530-0277.2008.00864.x.
Li L, Coles C, Lynch M, Hu X. Voxelwise and skeleton-based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. NeuroImage. 2009:S47. https://doi.org/10.1016/s1053-8119(09)70073-4.
Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1671–89. https://doi.org/10.1111/j.1530-0277.2009.01004.x.
Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30:10985–90. https://doi.org/10.1523/JNEUROSCI.5122-09.2010.
Chavarria MC, Sánchez FJ, Chou Y-Y, Thompson PM, Luders E. Puberty in the corpus callosum. Neuroscience. 2014;265:1–8. https://doi.org/10.1016/j.neuroscience.2014.01.030.
Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, et al. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2008;32:1732–40. https://doi.org/10.1111/j.1530-0277.2008.00750.x.
Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–202. https://doi.org/10.1111/j.1530-0277.1995.tb01600.x.
Fan J, Meintjes EM, Molteno CD, Spottiswoode BS, Dodge NC, Alhamud AA, et al. White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Hum Brain Mapp. 2015;36:2470–82. https://doi.org/10.1002/hbm.22785.
Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2010;34:354–63. https://doi.org/10.1111/j.1530-0277.2009.01097.x.
Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci. 2013;33:10098–109. https://doi.org/10.1523/JNEUROSCI.5004-12.2013.
Biffen SC, Dodge NC, Warton CMR, Molteno CD, Jacobson JL, Meintjes EM, et al. Compromised interhemispheric transfer of information partially mediates cognitive function deficits in adolescents with fetal alcohol syndrome. Alcohol Clin Exp Res. 2022. https://doi.org/10.1111/acer.14795.
Figley CR, Uddin MN, Wong K, Kornelsen J, Puig J, Figley TD. Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front Neurosci. 2021;15:799576. https://doi.org/10.3389/fnins.2021.799576.
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–16. https://doi.org/10.1016/j.neuroimage.2012.03.072.
Kamiya K, Hori M, Aoki S. NODDI in clinical research. J Neurosci Methods. 2020;346:108908. https://doi.org/10.1016/j.jneumeth.2020.108908.
Mah A, Geeraert B, Lebel C. Detailing neuroanatomical development in late childhood and early adolescence using NODDI. PLoS One. 2017;12:e0182340. https://doi.org/10.1371/journal.pone.0182340.
Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–91. https://doi.org/10.1093/cercor/10.10.981.
Young JM, Vandewouw MM, Mossad SI, Morgan BR, Lee W, Smith ML, et al. White matter microstructural differences identified using multi-shell diffusion imaging in six-year-old children born very preterm. Neuroimage Clin. 2019;23:101855. https://doi.org/10.1016/j.nicl.2019.101855.
Yang Y, Phillips OR, Kan E, Sulik KK, Mattson SN, Riley EP, et al. Callosal thickness reductions relate to facial dysmorphology in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2012;36:798–806. https://doi.org/10.1111/j.1530-0277.2011.01679.x.
Suttie M, Wozniak JR, Parnell SE, Wetherill L, Mattson SN, Sowell ER, et al. Combined face-brain morphology and associated neurocognitive correlates in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2018;42:1769–82. https://doi.org/10.1111/acer.13820.
Mullen EM, et al. Mullen scales of early learning. Circle Pines: AGS; 1995.
Bauer PJ. Recalling past events: from infancy to early childhood. Ann Child Dev. 1995;11:3.
Bauer PJ. Development of memory in early childhood. In: The development of memory in childhood; 1997. p. 83–111. Available: https://books.google.com/books?hl=en&lr=&id=u50m2oL0EdMC&oi=fnd&pg=PA83&dq=Bauer+PJ+1997&ots=-UdH_NwLCN&sig=Qt4aNMTkJOrb-ZC6pnjalxT0EsY.
Achenbach TM, Edelbrock C. Child behavior checklist. Burlington (Vt). 1991;7:371–92.
Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. https://doi.org/10.1542/peds.2004-0259.
Wechsler D, Pearson Education, Inc., Psychological Corporation. Wechsler intelligence scale for children - 5th edition. San Antonio: NCS Pearson, Inc.; 2014.
Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). San Antonio: Harcourt Assessment, Inc.; 2001.
Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 2012;68:389–99. https://doi.org/10.1002/mrm.23228.
Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, et al. Extending the Human Connectome Project across ages: imaging protocols for the lifespan development and aging projects. Neuroimage. 2018;183:972–84. https://doi.org/10.1016/j.neuroimage.2018.09.060.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–24. https://doi.org/10.1016/j.neuroimage.2013.04.127.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9931269.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10984517.
Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011;5:23. https://doi.org/10.3389/fninf.2011.00023.
Daducci A, Canales-Rodríguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran J-P. Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. Neuroimage. 2015;105:32–44. https://doi.org/10.1016/j.neuroimage.2014.10.026.
R Core Team. R: a language and environment for statistical computing. Vienna; 2021. Available: URL https://www.R-project.org/
Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge Academic; 1988.
Holm S. A simple sequentially rejective multiple test procedure. Scand Stat Theory Appl. 1979;6:65–70.
Giacalone M, Agata Z, Cozzucoli PC, Alibrandi A. Bonferroni-Holm and permutation tests to compare health data: methodological and applicative issues. BMC Med Res Methodol. 2018. https://doi.org/10.1186/s12874-018-0540-8.
Roid GH. Stanford Binet Intelligence Scales. 5th ed. Itasca: Riverside; 2003.
Li Q, Guo-Ross S, Lewis DV, Turner D, White AM, Wilson WA, et al. Dietary prenatal choline supplementation alters postnatal hippocampal structure and function. J Neurophysiol. 2004;91:1545–55. https://doi.org/10.1152/jn.00785.2003.
Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18:545–7. https://doi.org/10.1096/fj.03-0877fje.
Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, et al. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr. 2013;98:403–12. https://doi.org/10.3945/ajcn.112.040766.
Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci. 2014;1308:89–106. https://doi.org/10.1111/nyas.12314.
Best JR, Miller PH, Jones LL. Executive functions after age 5: changes and correlates. Dev Rev. 2009;29:180–200. https://doi.org/10.1016/j.dr.2009.05.002.
Karr JE, Areshenkoff CN, Rast P, Hofer SM, Iverson GL, Garcia-Barrera MA. The unity and diversity of executive functions: a systematic review and re-analysis of latent variable studies. Psychol Bull. 2018;144:1147–85. https://doi.org/10.1037/bul0000160.
Hartung J, Engelhardt LE, Thibodeaux ML, Harden KP, Tucker-Drob EM. Developmental transformations in the structure of executive functions. J Exp Child Psychol. 2020;189:104681. https://doi.org/10.1016/j.jecp.2019.104681.
Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. https://doi.org/10.1016/s0896-6273(01)00583-9.
Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–68. https://doi.org/10.1016/j.neuroimage.2013.12.044.
Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48:2496–508. https://doi.org/10.1016/j.neuropsychologia.2010.04.024.
Zelazo PD. Executive function and psychopathology: a neurodevelopmental perspective. Annu Rev Clin Psychol. 2020;16:431–54. https://doi.org/10.1146/annurev-clinpsy-072319-024242.
Mattson SN, Bernes GA, Doyle LR. Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol Clin Exp Res. 2019;43:1046–62. https://doi.org/10.1111/acer.14040.
de Water E, Krueger AM, Lindgren CW, Fuglestad AJ, Rockhold MN, Sandness KE, et al. Early delay of gratification predicts later inhibitory control and academic performance in children with prenatal alcohol exposure. Child Neuropsychol. 2021;27:109–24. https://doi.org/10.1080/09297049.2020.1798372.
Rockhold MN, Krueger AM, de Water E, Lindgren CW, Sandness KE, Eckerle JK, et al. Executive and social functioning across development in children and adolescents with prenatal alcohol exposure. Alcohol Clin Exp Res. 2021;45:457–69. https://doi.org/10.1111/acer.14538.
Delis DC, Kaplan E, Kramer JH. Delis - Kaplan executive function system: examiner’s manual. San Antonio: Psychological Corporation; 2001.
Suchy Y, Mullen CM, Brothers S, Niermeyer MA. Interpreting executive and lower-order error scores on the timed subtests of the Delis-Kaplan Executive Function System (D-KEFS) battery: error analysis across the adult lifespan. J Clin Exp Neuropsychol. 2020:982–97. https://doi.org/10.1080/13803395.2020.1832203.
Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, et al. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci. 2008:1313–9. https://doi.org/10.1523/jneurosci.5067-07.2008.
Fan J, Jacobson SW, Taylor PA, Molteno CD, Dodge NC, Stanton ME, et al. White matter deficits mediate effects of prenatal alcohol exposure on cognitive development in childhood. Hum Brain Mapp. 2016;37:2943–58. https://doi.org/10.1002/hbm.23218.
Ma X, Coles CD, Lynch ME, Laconte SM, Zurkiya O, Wang D, et al. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–22. https://doi.org/10.1097/01.alc.0000171934.22755.6d.
Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6 Available: https://www.ncbi.nlm.nih.gov/pubmed/2081237.
Petrenko CLM, Alto ME, Hart AR, Freeze SM, Cole LL. “I’m doing my part, I just need help from the community”: intervention implications of foster and adoptive parents’ experiences raising children and young adults with FASD. J Fam Nurs. 2019:314–47. https://doi.org/10.1177/1074840719847185.
Acknowledgements
We thank the children and families who participated in this research. We acknowledge the contributions of Proof Alliance (formerly the Minnesota Organization on Fetal Alcohol Spectrum Disorders) which include assistance with participant recruitment and public awareness of the study.
Funding
This work was supported by the National Institutes of Health (NIH) grants R21AA019580, R33AA019580, R01AA024123, P41EB027061, P30NS076408, and 1S10OD017974.
Author information
Authors and Affiliations
Contributions
BAG participated in the analysis of the study as well as the writing of the manuscript. MEA participated in the analysis of the study and the writing of the manuscript. AME participated in the data collection, analysis of the study, and the writing of the manuscript. DJR participated in the design and conduct of the study including neuroimaging data acquisition, neuroimaging data inspection and analysis, and the writing of the manuscript. EdW participated in the writing of the manuscript. JKE participated in the design and execution of the study including data collection and study procedures as well as the writing of the manuscript. JPR participated in the design and conduct of the study and the preparation of the study drug as well as the writing of the manuscript. BAM participated in the design and conduct of the study including neuroimaging data acquisition, neuroimaging data inspection and analysis, and the writing of the manuscript. AJF participated in the design, execution, and analysis of the study as well as the writing of the manuscript. SHZ participated in the design of the study and the writing of the manuscript. MKG participated in the design of the study as well as the writing of the manuscript. JRW participated in the design, execution, and analysis of the study as well as the writing of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All aspects of the study were approved by the University of Minnesota IRB, and all participants’ parents participated in a comprehensive informed consent procedure and signed consent forms.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gimbel, B.A., Anthony, M.E., Ernst, A.M. et al. Long-term follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder: corpus callosum white matter microstructure and neurocognitive outcomes. J Neurodevelop Disord 14, 59 (2022). https://doi.org/10.1186/s11689-022-09470-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11689-022-09470-w