Abstract
Background
Individuals with premutation alleles of the fragile X mental retardation 1 (FMR1) gene are at risk of developing fragile X-associated tremor/ataxia syndrome (FXTAS) during aging. Characterization of motor issues associated with aging in FMR1 premutation carriers is needed to determine neurodegenerative processes and establish new biobehavioral indicators to help identify individuals at greatest risk of developing FXTAS.
Methods
We examined postural stability in 18 premutation carriers ages 46–77 years and 14 age-matched healthy controls. Participants completed a test of static stance and two tests of dynamic postural sway on a force platform to quantify postural variability and complexity. CGG repeat length was measured for each premutation carrier, and MRI and neurological evaluations were conducted to identify carriers who currently met criteria for FXTAS. Of the 18 premutation carriers, seven met criteria for definite/probable FXTAS (FXTAS+), seven showed no MRI or neurological signs of FXTAS (FXTAS−), and four were inconclusive due to insufficient data.
Results
Compared to controls, premutation carriers showed increased center of pressure (COP) variability in the mediolateral (COPML) direction during static stance and reduced COP variability in the anterior-posterior (COPAP) direction during dynamic AP sway. They also showed reductions in COPML complexity during each postural condition. FXTAS+ individuals showed reduced COPAP variability compared to FXTAS− carriers and healthy controls during dynamic AP sway. Across all carriers, increased sway variability during static stance and decreased sway variability in target directions during dynamic sways were associated with greater CGG repeat length and more severe neurologically rated posture and gait abnormalities.
Conclusion
Our findings indicate that aging FMR1 premutation carriers show static and dynamic postural control deficits relative to healthy controls implicating degenerative processes of spinocerebellar and cerebellar-brainstem circuits that may be independent of or precede the onset of FXTAS. Our finding that FXTAS+ and FXTAS− premutation carriers differed on their level of intentional AP sway suggests that neural mechanisms of dynamic postural control may be differentially impacted in patients with FXTAS, and its measurement may be useful for rapidly and precisely identifying disease presence and onset.
Similar content being viewed by others
Background
Mutations of the fragile X mental retardation 1 (FMR1) gene involving > 200 cytosine-guanine-guanine (CGG) repeat expansions in the 5′-untranslated region cause fragile X syndrome is the leading inherited cause of intellectual disability. FMR1 premutations characterized by 55–200 CGG repeats are associated with subclinical psychiatric, cognitive, and motor issues [1, 2]. Approximately one third of aging premutation carriers also develop fragile X-associated tremor/ataxia syndrome (FXTAS), a progressive neurodegenerative disorder characterized by kinetic tremor, gait ataxia, and Parkinsonism and involves neurodegenerative processes of spinocerebellar and cerebellar-brainstem circuits [1,2,3,4]. Penetrance of FXTAS among premutation carriers increases with age, and the onset of motor deficits typically occurs after 50 years of age with subsequent rapid neurological and cognitive decline [1].
Increased CGG repeat length is associated with increased risk of developing FXTAS [4,5,6,7,8]. Cerebellar lesions and cerebral atrophy are common [3, 4] and serve as part of the diagnostic criteria for FXTAS [1, 2, 9]. However, FXTAS often is misdiagnosed due to its clinical overlap with other neurodegenerative disorders (e.g., Parkinson’s disease), especially early in its course [10, 11]. Precise approaches for quantifying neurodegenerative processes associated with FMR1 premutations across behavioral, neural, and genetic levels are needed to advance our understanding of the cause of the disease, identify prodromal signs, and monitor disease progression and treatment outcomes.
Spinocerebellar and cerebellar-brainstem function often is measured behaviorally using tests of static postural control during which participants stand as still as possible, and dynamic postural control in which individuals initiate continuous body sway along an axis. Paramedian cerebellar lobules including the vermis receive afferent input both from motor and posterior parietal cortices as well as direct innervation from the spinal cord [12]. Spinocerebellar inputs provide rapid proprioceptive feedback information that can be integrated with somatosensory, visual and vestibular feedback in cerebellar-cortical networks to maintain postural stability. Tests of static and dynamic stances have been used to index cerebellar dysfunctions in individuals with Friedreich’s ataxia [13], psychiatric disorders involving cerebellar-brainstem dysfunction [14], and movement disorders such as Parkinson’s disease [15]. In the present study, we investigated static and dynamic postural control in aging FMR1 premutation carriers in order to characterize cerebellar-dependent motor processes.
Multiple prior studies have examined postural control in aging FMR1 premutation carriers, but these studies have suggested that postural control is relatively intact in carriers who showed no signs of FXTAS [5, 10, 16]. Importantly, each prior study of premutation carriers quantified postural control using individuals’ postural sway area, which is an aggregate measure of body sway in both anterior-posterior (AP) and mediolateral (ML) directions. As increased sway variability in ML directions predicts the risk of fall in older adults [17,18,19] and is used to monitor the progression of cerebellar ataxia [13] and Parkinson’s disease [15, 20], precise quantification of postural sway in ML directions may be more sensitive to atypical neurodegenerative processes. Analyses separating center of pressure (COP) variability in ML and AP directions are needed to determine if postural control mechanisms are disrupted during aging in FMR1 premutation carriers.
Assessments of dynamic postural sways in which participants continuously move their body along an axis (e.g., AP or ML) provide important information about individuals’ ability to reactively refine postural sway amplitude and velocity to maintain balance. During dynamic sway, gravitational torque increases due to increases in sway amplitude in the target direction. Increased demand to reverse postural sway acceleration prior to the body’s center of mass approaching the base of support boundary makes dynamic sway more challenging to control than static stance [21, 22]. In contrast to static stance, postural instability during dynamic stance is reflected by COP variability reductions in target directions that may compensate for reduced postural control as individuals attempt to avoid moving their body’s center of mass close to their base of support boundary [23]. No known studies have examined dynamic postural control in aging FMR1 premutation carriers.
Measurement of non-linear time-dependent properties of individuals’ postural sway also may provide sensitive indices of the integrity of the postural control system [24]. Postural instability can be examined by quantifying the complexity of the long-range correlation of the COP time series along multiple temporal scales using the measure of detrended fluctuation analysis (DFA) [25, 26]. DFA assumes that postural sway involves a combination of deterministic and stochastic process [25,26,27]. Deterministic processes represent a “stable” state allowing individuals to sway within their base of support in a predictable manner (i.e., the regularity of postural sway). Stochastic processes reflect the integration of individuals’ internal state (e.g., proprioception) and processing of external feedback (e.g., visual, vestibular, and somatosensory) which affords flexibility in controlling postural sway relative to task demands (i.e., the complexity of the COP time series). Neurodegeneration of the cerebellum is associated with a reduction in the ability to integrate these spontaneous processes and a resulting reduction of COP complexity [14, 26]. Consistent with this idea, reduced postural sway complexity during static stance has been documented in studies of aging [26], cerebellar atrophy [14], and Parkinson’s disease [28]. Yet, the extent to which COP complexity is affected in FMR1 premutation carriers remains unknown.
In the present study of FMR1 premutation carriers, we examined postural control variability and complexity in both AP and ML directions across static and dynamic stances. All premutation carriers completed neurological testing to determine whether they showed clinical or radiological signs of FXTAS. We hypothesized that FMR1 premutation carriers would show increased COP variability during static stance compared to healthy aging controls, with the effect more pronounced in the ML direction. During dynamic postural sways, we predicted that premutation carriers would show reduced COP variability in target directions compared to controls. For all standing postures, we hypothesized reduced COP complexity in individuals with FMR1 premutations compared to controls. We also predicted that the severity of postural sway deficits in premutation carriers would be related to increased CGG repeats.
Based on the neurological and MRI testing, we also characterized premutation carriers as either having probable/definite (FXTAS+) or no signs of FXTAS (FXTAS−). We expected that the FXTAS+ subgroup would show reduced postural control relative to FXTAS− carriers and controls.
Methods
Participants
Eighteen FMR1 premutation carriers were identified through our fragile X clinics and postings on local and national fragile X association listservs. Fourteen controls matched on age and sex were recruited through community advertisements. During an initial screening interview, no premutation carriers reported any issues of tremor or ataxia. They completed genetic testing to quantify CGG repeat length, a T2-weighted MRI scan and a structured neurological evaluation conducted by a clinical neurologist (PK) using the International Cooperative Ataxia Rating Scale (ICARS) [29]. All participants completed the abbreviated battery of the Stanford-Binet Intelligence Scales, Fifth Edition [30] to characterize cognitive abilities, including nonverbal fluid reasoning and verbal knowledge (Table 1). Participants then completed tests of static and dynamic postural stances. All study procedures were approved by the local IRB.
Both FMR1 premutation carriers and control participants were excluded if they reported lower extremity orthopedic surgery within the past year, or any musculoskeletal disorder that could potentially cause atypical postural or gait functioning, or a history of medications known to affect motor functioning [31]. Eight participants (seven premutation carriers, one control) reported being on medication within 48 h of testing, including antidepressants (selective serotonin reuptake inhibitors: wo premutation carriers, one control who reported taking medication for premenstrual syndrome; serotonin-norepinephrine reuptake inhibitors: two premutation carriers), sedatives/hypnotics (benzodiazepine anxiolytic: one premutation carrier; nonbenzodiazepine anxiolytic: one premutation carrier), synthroids (three premutation carriers), or a mood stabilizer (one premutation carrier).
Procedure and approach
CGG repeat length
FMR1 CGG repeat length was quantified for all premutation carriers. Molecular testing was conducted at Dr. Berry-Kravis’ Molecular Diagnostic Laboratory at Rush University. Genomic DNA was isolated from peripheral blood leukocytes samples. The FMR1 polymerase chain reaction (PCR) test with quantification of allele-specific CGG repeat length was performed using commercially available kits (Asuragen, Inc., Austin, TX).
T2-weighted magnetic resonance imaging (MRI) scan
FMR1 premutation carriers underwent a T2-weighted MRI scan (repetition time = 6350 msec; echo time = 100 msec; flip angle = 120°; field of view = 256 × 156 × 256 mm3; 78 axial slices; voxel size = 1 mm2 × 2 mm; no gap) to test for the presence of hyperintensities within the middle cerebellar peduncle (i.e., the MCP sign), cerebral atrophy, or other cerebral or cerebellar-brainstem alterations associated with FXTAS [2,3,4]. T2-weighted scans were analyzed by a trained neuroradiologist (SL) with expertise in diseases of aging.
Neurological examination
FMR1 premutation carriers completed a structured neurological exam administered by a neurologist (PK) with expertise in ataxia and movement disorders in aging. This exam included evaluations of movement and gait as well as administration of the ICARS. The ICARS is comprised of 19 sections examining postural and gait disturbances, ataxia, dysarthria, and oculomotor functions. Higher scores indicate a higher level of cerebellar ataxia. The ICARS has been validated previously for diagnosis in patients with focal cerebellar lesions [32] and Friedrich’s ataxia [33].
Postural control assessments
Postural stability was assessed using an AMTI (American Mechanical Technology, Inc., Watertown, MA) AccuGait strain gauge force platform (size 49.78 × 49.78 cm) with a sampling rate of 1000 Hz. All participants completed tests of static and dynamic stances by standing on the platform with bare feet shoulder-width apart and arms resting at their sides. Participants’ foot positions were outlined on a piece of tracing paper placed on top of the force platform prior to the first trial to ensure consistent placement and orientation of the feet during each trial. During the static stance test, participants were instructed to stand as still as possible for three 30-s trials. During dynamic stance tests, participants completed three 30-s trials for each of two different self-initiated postural sways—AP and ML. For each dynamic stance condition, participants were instructed to sway continuously in the target direction at a comfortable speed and amplitude without raising their toes or heels. Each stance trial was followed by 30 s of rest. Nine trials (three conditions × three trials) were examined in total. Order of administration of the static and dynamic stance tests and the two directions of the dynamic stance test were counterbalanced across participants.
The force and moment data collected from the force plate were down sampled to 200 Hz and low pass filtered using a fourth-order double pass Butterworth filter with a cutoff frequency of 6 Hz in Matlab 2017a (MathWorks, Inc., Natick, MA). The COP time series were derived from the force and moment data for each standing posture [22]. The variability of individuals’ postural sway was quantified using COP standard deviation in both the AP (COPAP) and ML (COPML) directions as we did previously [34].
The complexity of individuals’ postural sway in both directions was quantified using the α exponent of DFA [25, 26]. DFA is a non-linear measurement quantifying the pattern of variation of a time series across multiple time scales [25, 26]. Its computation is based on the assumption that variations present in a system due to intrinsic dynamics exhibit fractal properties of long-range correlations (see Appendix for the detailed algorithm). In brief, the α exponent of DFA varies between 0 and 2 (i.e., 0 < α < 2) including four ranges of values separated at 0.5, 1, 1.5, and 2. When 0 < α < 0.5 or 1 < α < 1.5, the time series is anti-correlated with a smaller α representing increased anti-correlation and complexity of the signal. When 0.5 < α < 1 or 1.5 < α < 2, increased alpha represents increased long-range correlation and reduced complexity of the time series. The COP time series consist of 6000 data points (30 s × 200 data points/s), which has been shown to be sufficient for analyses of DFA [35].
Statistical analyses
The COP standard deviation and α exponent of DFA were averaged across trials and compared between groups (FMR1 premutation carriers vs. controls) using separate repeated measures ANOVAs including stance condition (static vs. AP sway vs. ML sway) and COP direction (AP vs. ML) as within subjects factors. The Greenhouse–Geisser estimate was used to provide a conservative test of ANOVA main and interaction effects for all repeated measure ANOVAs in which Mauchly’s test indicated a violation of sphericity. Statistically significant interaction effects were probed using Bonferroni corrected post-hoc analyses. All assumptions of normality and homogeneity of variance were verified for each measure of postural control.
Pearson correlations were conducted to determine the inter-relationships of posture variables found to be significantly different between groups in our main analyses and CGG repeat length. Due to the non-normal distributions of ICARS scores, Spearman correlations were applied to examine the relationships between COP-dependent variables and ICARS posture and gait subscale and total scores. The relationships between CGG repeat length and each ICARS subscale score and ICARS total scores also were examined using Spearman correlations. Correlations were interpreted as significant if ∣r∣ > 0.5.
Based on the MRI and ICARS evaluations, premutation carriers were identified as having (FXTAS+) or not having FXTAS (FXTAS−) according to published clinical criteria [1, 2, 9]. FXTAS+ individuals (n = 7) included premutation carriers with one major MRI sign plus one major neurological sign (definite FXTAS) and those with either one major MRI sign plus one minor neurological sign or those with two major neurological signs (probable FXTAS). FXTAS− individuals included premutation carriers with no MRI or clinical signs of FXTAS (N = 7). There was no difference between FXTAS+ and FXTAS− subgroups in the number of medications reported. Four premutation carriers who failed to complete the neurological evaluation due to scheduling issues (N = 2) or MRI scan due to claustrophobia (N = 2) were excluded from analyses comparing FXTAS+ individuals, FXTAS− individuals, and controls. Separate Kruskal–Wallis tests were performed on COP standard deviation and α exponent of DFA measures to examine group differences (FXTAS+ vs. FXTAS− vs. healthy controls). One-way ANOVAs also were performed to compare age and CGG repeat length between FXTAS+ and FXTAS− individuals. All results were interpreted as significant if p < 0.05.
Results
Postural sway variability in FMR1 premutation carriers and healthy controls
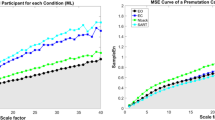
COP standard deviation was greater during dynamic stances compared to static stance (stance condition main effect: F1.304, 28.991 = 643.203, p < 0.001; Fig. 1a). COP standard deviation was greater in the AP than in the ML direction during static stance, whereas it was greater in the target direction during dynamic stances (stance condition × direction interaction effect: F1.115, 28.991 = 933.867, p < 0.001). The interaction effect of stance condition, direction, and group was significant (F1.115, 28.991 = 6.082, p = 0.017). Relative to controls, FMR1 premutation carriers showed increased COPML standard deviation during static stance (FMR1 − controls = 0.071 cm, SE = 0.034 cm with F1,26 = 4.334, p = 0.047). During dynamic AP postural sway, premutation carriers showed lower COP standard deviation in the target directions compared to healthy controls (FMR1 − controls = − 0.668 cm, SE = 0.245 cm with F1,26 = 7.435, p = 0.011). Premutation carriers also showed less COP sway in the target direction during the ML condition, although this effect did not reach statistical significance (FMR1 − controls = − 1.157 cm, SE = 0.604 cm with F1,26 = 3.663, p = 0.067).
a Center of pressure (COP) standard deviation in the anterior-posterior (AP) and mediolateral (ML) directions. b The α exponent of detrended fluctuation analysis (DFA) of COP time series in both directions are shown as a function of standing condition. FMR1 stands for FMR1 premutation carriers. Between-group differences are marked as * at 0.05 level and ** at 0.01 level. Error bars represent standard error
Postural sway complexity in FMR1 premutation carriers and healthy controls
The α exponent of COPAP and COPML showed significant increases during dynamic stances compared to the static stance condition (stance condition main effect: F1.645, 54,837 = 73.166, p < 0.001; Fig. 1b). The α exponent was greater in the AP direction compared to the ML direction during static stance (stance condition × direction interaction effect: F1.891, 54,837 = 121.022, p < 0.001; static stance: COPAP− COPML = 0.108, SE = 0.016 with p < 0.001), while it was greater in the target directions during both dynamic stances (dynamic AP sway: COPAP − COPML = 0.120, SE = 0.012 with F1, 26 = 104. 534, p < 0.001; dynamic ML sway: COPML − COPAP = 0.131, SE = 0.013 with F1, 26 = 111.511 p < 0.001). FMR1 premutation carriers showed a greater α exponent in the ML direction than control participants across all standing conditions reflecting reduced complexity of their COP time series (stance condition × direction × group interaction: F1, 58 = 7.572, p = 0.010; static stance: FMR1-controls = 0.062, SE = 0.027, F1, 26 = 16.622, p < 0.001; dynamic AP sway: FMR1-controls = 0.054, SE = 0.019, F1, 26 = 24.905, p < 0.001; dynamic ML sway: FMR1-controls = 0.045, SE = 0.024, F1, 26 = 4.308, p < 0.05).
Postural sway in FXTAS+ and FXTAS− carriers
Table 2 summarizes CGG repeat length, radiological and neurological results for each FMR1 premutation carrier. Results from the Kruskal-Wallis test showed a group main effect of COPAP standard deviation (χ2(2) = 12.112, p = .002) during dynamic AP sway (Fig. 3) characterized by reduced COPAP variability in FXTAS+ individuals compared to FXTAS− individuals (χ2(1) = 7.547, p = .006) and control participants (χ2(1) = 10.776, p = .001). No differences in COP variability for any direction or stance were found between FXTAS− and healthy control participants (χ2(1) = .050, p = .823). One-way ANOVAs performed on age (F 1,12 = .733, p = .409) and CGG repeat length (F 1,12 = 1.612, p = .228) showed no difference between FXTAS+ and FXTAS− individuals.
Clinical associations
Greater COP variability in both the AP and ML directions during static stance was associated with higher CGG repeats in FMR1 premutation carriers (Table 3; Fig. 2). Lower sway variability in target directions during dynamic AP sway also was associated with higher CGG repeats in premutation carriers. Greater α exponent of DFA in target directions during dynamic AP postural sway was related to higher CGG repeats. Lower COP variability in the AP direction during dynamic AP sway was associated with higher ICARS posture and gait subscale and ICARS total scores in premutation carriers. None of the ICARS subscale scores were associated with CGG repeat length (dysarthria: r = − 0.090, p = 0.742; kinetic ataxia: r = 0.003, p = 0.991; oculomotor disorders: r = − 0.238, p = 0.374; gait and posture: r = 0.010, p = 0.972; total: r = 0.088, p = 0.745). CGG repeat length was not associate with any postural control variables or ICARS scores within the FXTAS+ or FXTAS− subgroups (Additional files 1 and 2: Tables S1 and S2).
Scatter plots of significant statistical correlations presented in Table 3. Data were color-coded based on the diagnostic classification of each individual FMR1 premutation carrier. ICARS scores were missing for two inconclusive individuals due to scheduling issues
Discussion
In the present study, we provide new evidence that during middle to late adulthood, FMR1 premutation carriers show reduced postural stability that is related to larger CGG repeat expansions, and thus covaries with FXTAS disease risk [5,6,7,8]. Four key findings are highlighted. First, relative to controls, FMR1 premutation carriers showed greater postural sway variability in the ML direction during static stance and reduced postural sway along the target direction during intentional AP sway suggesting that spinocerebellar and cerebellar-brainstem circuits supporting postural stability are disrupted in aging FMR1 premutation carriers (Fig. 1a). Second, premutation carriers demonstrated reduced complexity of their COPML time series across all standing conditions implicating deficits in their ability to dynamically adapt to inherent postural perturbations (Fig. 1b). Third, both COP variability and complexity alterations in FMR1 premutation carriers were associated with higher CGG repeats and ICARS-rated posture, gait, and motor deficits, suggesting that our measures may provide sensitive and highly quantifiable biologically based markers of behavioral and neurological features associated with FXTAS risk or progression in premutation carriers (Fig. 2). Last, FXTAS+ individuals showed reduced AP postural sway variability during dynamic AP trials relative to carriers without clinical signs of FXTAS (FXTAS−) and healthy controls suggesting that mechanisms supporting intentional sway may be selectively disrupted in FXTAS (Fig. 3). Our measure of dynamic AP sway thus may be useful for rapidly and precisely differentiating premutation carriers with and without FXTAS.
Scatter plot of COP standard deviation in AP directions during dynamic AP sway. Data are color-coded based on the diagnostic classification of FMR1 premutation carriers and healthy controls. Box plots show (left to right) the minimum (cap), first quartile, median, third quartile, and maximum (cap) values of each group. Two data points in the control group were located outside of the 1.5× inter-quartile range
Postural control deficits in FMR1 premutation carriers during static stance
Postural control is a continuous process during which individuals actively align their body’s center of mass within their base of support area in response to inherent noise and changes in environmental (e.g., moving to a slippery surface) and task (e.g., when leaning forward to reach an object; when moving from sitting to standing) demands [21]. Increases in postural sway variability are common in aging adults and reflect neurodegenerative processes involving decreased nerve conduction velocity, deterioration of visual, vestibular and somatosensory feedback systems, reduced muscle strength, and degeneration of central modulation of motoneuron pools [21, 36]. However, more severe increases in postural sway variability and reductions in complexity can reflect pathology of the cerebellum [13], basal ganglia [15], or cortical motor areas [25]. In the context of documented reductions of cerebellar and brainstem volumes [4], deterioration of cerebellar white matter microstructure [3], and increased rates of cerebellar cellular intranuclear inclusions [37] in individuals with FXTAS, our results of greater COPML variability (Fig. 1a) and reduced COPML complexity (Fig. 1b) in premutation carriers implicate spinocerebellar and cerebellar-brainstem processes independent of or prior to the onset of FXTAS. It is possible that similar deficits are evident earlier in life in premutation carriers and thus are not reflective of neurodegenerative processes, but instead motor control issues related to the premutation allele. Although one prior study [38] indicated that postural control is relatively intact during middle adulthood in female premutation carriers, direct comparisons of younger adult premutation carriers and controls on our measures are needed to determine whether postural control deficits represent atypical neurodevelopmental or neurodegenerative processes or both.
Postural control deficits in FMR1 premutation carriers during dynamic postural sways
During dynamic postural sways, premutation carriers showed reduced COP variability in target directions compared to healthy aging adults. When aging individuals intentionally lean in one direction, they often present a reduced ability to approach their base of support boundaries [39]. This behavioral change during aging servers as a compensatory mechanism for individuals’ reduced ability to maintain stability or recover from shifts in their COP [21]. Our results suggest this compensatory mechanism is utilized to an even greater degree by aging individuals with FMR1 premutation alleles as they counterbalanced their postural instability by minimizing body movements away from the neutral position during dynamic stances (Fig. 1a). Results indicating that reduced dynamic sway is associated with longer CGG repeat length (Fig. 2) implicate more severe spinocerebellar or cerebellar-brainstem dysfunctions or degeneration for those individuals with greater CGG repeats. Given that greater CGG repeat length confers increased risk for the development of FXTAS, our findings also indicate that reduced dynamic sway variability may represent an early behavioral marker of FXTAS disease risk and progression.
Pathological postural sway is characterized by increased local stability and reduced complexity of the COP time series [24]. Reduced postural sway complexity in premutation carriers implicates deficient central integration of sensory feedback processes, movement anticipation, motor planning, and systems supporting coordinated musculoskeletal execution of motor commands [25, 26]. Our findings of reduced COPML complexity ( α exponent of DFA measure was within the range from 1.5 to 2 indicating increased long-range correlation and reductions in COPML complexity) in premutation carriers are consistent with previous studies documenting progressive decay of lateral postural sway associated with spinocerebellar and cerebellar-brainstem circuitry decline in Friedreich’s ataxia [13]. Evidence of reductions in COP variability in target directions and in stochastic processes of lateral sway in FMR1 premutation carriers each suggest atypical deterioration of dynamic postural control mechanisms involved in modulating center of mass movement in relation to the base of support [21, 34] .
FXTAS specific deficits of postural control
Subgroup analyses of carriers with and without FXTAS suggested that reductions in dynamic AP sway variability are specific to FXTAS+ individuals and are largely absent in FXTAS− premutation carriers (Fig. 3). Notably, FXTAS+ and FXTAS− individuals showed minimal overlap in their level of dynamic AP sway variability suggesting this measure may provide a highly sensitive and specific index of FXTAS risk or progression. COPAP during dynamic AP sway also was the only measure of postural control that was associated with greater CGG repeat length and ICARS posture and gait subscale and total scores (Table 3) in premutation carriers, suggesting that it represents a highly quantifiable biobehavioral indicator of the presence of or risk for FXTAS. These findings are consistent with the well-documented cerebellar pathology present in many FXTAS patients and the cerebellum’s known role in postural control [3, 4]. Our dynamic postural control tests provide significant advantages over current clinical and neurological evaluations for identifying FXTAS as they are highly precise and efficient (e.g., they require 3–5 min to administer). In combination with genetic and MRI exams, dynamic postural control variability in the AP direction may serve as a reliable marker identifying the presence of FXTAS or helping to guide clinical assessments and screening. While further testing is needed to determine both sensitivity and specificity of our measures across a larger number of aging premutation carriers and assessing their utility across male and female premutation carriers who may present with different symptoms associated with FXTAS [1, 40], our results suggest reduced dynamic AP sway may be a rapid and precise measure useful for identifying FXTAS in aging premutation carriers.
Neurobiological mechanisms underlying postural control deficits in aging FMR1 premutation carriers
Our results document that increased CGG repeats are associated with increased postural sway variability in both the AP and ML directions during static stance and decreased sway variability during dynamic AP sway among premutation carriers (Table 3; Fig. 2). Previous studies suggest that higher CGG repeats are associated with increased risk for and severity of FXTAS [6,7,8, 41]. At the molecular level, the CGG premutation allele results in increased FMR1 mRNA levels and, in some cases, decreases in fragile X mental retardation protein (FMRP) [42, 43]. Increased FMR1 mRNA is linked to a cumulative cytotoxic effect associated with intranuclear inclusions observed in neuronal and astrocytic nuclei of the cerebellum and brainstem in postmortem tissue [37, 41]. FMRP plays an important role in RNA-binding translation and channel-binding regulation at synapses [44] affecting the formation of axons, myelination [45], and dendritic maturation [46]. Reductions of FMRP in premutation carriers could disrupt the microstructural integrity of white matter in the primary fiber pathways of the cerebellum, including the superior, middle, and inferior cerebellar peduncles, as well as other large white matter fiber tracts such as the corpus callosum [3]. These cellular and brain system alterations have been documented in individuals with FXTAS, including selective degeneration of the middle cerebellar peduncle—the primary input pathway of the cerebellum [2, 4, 9]. Importantly, cerebellar inputs from neocortical regions are critical to the cerebellum’s role in integrating internal and external sensory feedback information in order to dynamically calibrate motor output. Greater motor variability [34, 47, 48] and reduced sensorimotor complexity [49] each have been demonstrated in individuals with disorders affecting the cerebellum. The postural control deficits reported here in premutation carriers thus may provide important indices of cerebellar mechanisms contributing to clinical issues in individuals with FMR1 premutations.
Study limitations and future directions
While the present study documents several novel findings useful for identifying pre-clinical signs of FXTAS, our results should be considered in the context of a few limitations. First, longitudinal studies are needed to determine how measures of postural variability and complexity vary across ages in premutation carriers and whether postural control issues identified in this study are evident earlier in life in premutation carriers and thus are not reflective of the aging process associated with FMR1 premutation, but instead motor control issues related to downstream effects of premutation alleles. Second, FXTAS disproportionately affects males, and the nature of symptoms appear to vary across males and females due to X-inactivation effects [50, 51]. Future studies are needed to clarify postural control processes in larger samples of male and female aging premutation carriers and determine their course in individuals with and without FXTAS.
Conclusions
Our results suggest that increased postural sway variability during static stance, decreased body movement in target directions during dynamic stance, and decreased lateral sway complexity are present in aging FMR1 premutation carriers compared to controls and are associated with larger CGG repeat expansions. Importantly, we also find that FXTAS+ individuals show reduced COPAP variability during dynamic sway relative to FXTAS− carriers, suggesting that reductions in the ability to control AP sway may provide a highly quantifiable and rapid biobehavioral index of FXTAS. Taken together, these results indicate that FMR1 premutation carriers experience reduced postural control implicating cerebellar-brainstem circuits and that precision measures of postural control may provide useful indicators of FXTAS risk or progression and important markers of disease-related mechanisms.
Abbreviations
- AP:
-
Anterior-posterior
- CGG:
-
Cytosine-guanine-guanine
- COP:
-
Center of pressure
- COPAP :
-
Center of pressure in the anterior-posterior direction
- COPML :
-
Center of pressure in the mediolateral direction
- DFA:
-
Detrended fluctuation analysis
- FMR1 mRNA:
-
FMR1 messenger RNA
- FMR1 :
-
Fragile X mental retardation 1
- FMRP:
-
Fragile X mental retardation protein
- FXTAS:
-
Fragile X-associated Tremor/Ataxia syndrome
- ICARS:
-
International Cooperative Ataxia Rating Scale
- ML:
-
Mediolateral
- MRI:
-
Magnetic resonance imaging
References
Diener HC, Dichgans BJ, Bacher M, Gompf B. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol. 1984;57:134–42.
Narcisa V, Aguilar D, Nguyen DV, Campos L, Brodovsky J, White S, et al. A quantitative assessment of tremor and ataxia in female FMR1 premutation carriers using CATSYS. Curr Gerontol Geriatr Res. 2011;2011:484713. https://doi.org/10.1155/2011/484713.
Tabolacci E, Palumbo F, Nobile V, Neri G. Transcriptional reactivation of the FMR1 gene. A possible approach to the treatment of the fragile X syndrome. Genes (Basel). 2016;7(8). https://doi.org/10.3390/genes7080049.
Schmitt LM, Cook EH, Sweeney JA, Mosconi MW. Saccadic eye movement abnormalities in autism spectrum disorder indicate dysfunctions in both cerebellum and brainstem. Molecular Autism. 2014;5:47.
Birch RC, Hocking DR, Cornish KM, Menant JC, Georgiou-Karistianis N, Godler DE, et al. Preliminary evidence of an effect of cerebellar volume on postural sway in FMR1 premutation males. Genes Brain Behav. 2015;14(3):251–9. https://doi.org/10.1111/gbb.12204.
Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age Ageing. 2004;33(6):602–7. https://doi.org/10.1093/ageing/afh218.
Kilby MC, Slobounov SM, Newell KM. Aging and the recovery of postural stability from taking a step. Gait Posture. 2014a;40(4):701–6. https://doi.org/10.1016/j.gaitpost.2014.08.002.
Davis JK, Broadie K. Multifarious functions of the fragile X mental retardation protein. Trends Genet. 2017. https://doi.org/10.1016/j.tig.2017.07.008.
Tassone F, Adams J, Berry-Kravis EM, Cohen SS, Brusco A, Leehey MA, et al. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):566–9. https://doi.org/10.1002/ajmg.b.30482.
Hall DA, O'Keefe JA. Fragile X-associated tremor ataxia syndrome: the expanding clinical picture, pathophysiology, epidemiology and update on treatment. Other Hyperkinet Mov. 2012;2:1–11.
Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72(4):869–78. https://doi.org/10.1086/374321.
Shelton AL, Cornish KM, Godler DE, Bui QM, Kolbe S, Feilding J. White matter microstructure, cognition, and molecular markers in fragile x premutation carriers. Neurology. 2017;88(22):2080–8.
Aguilar D, Sigford KE, Soontarapornchai K, Nguyen DV, Adams PE, Yuhas JM, et al. A quantitative assessment of tremor and ataxia in FMR1 premutation carriers using CATSYS. Am J Med Genet A. 2008;146A(5):629–35. https://doi.org/10.1002/ajmg.a.32211.
Maki BE, Holliday PJ, Fernie GR. Aging and postural control: a comparison of spontaneous- and induced-sway balance tests. J Am Geriatr Soc. 1990;38:381–9.
Bryce RM, Sprague KB. Revisiting detrended fluctuation analysis. Sci Rep. 2012;2:315. https://doi.org/10.1038/srep00315.
Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain Cogn. 2008;68(3):415–35.
Roerdink M, De Haart M, Daffertshofer A, Donker SF, Geurts AC, Beek PJ. Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Exp Brain Res. 2006;174(2):256–69. https://doi.org/10.1007/s00221-006-0441-7.
Schmit JM, Riley MA, Dalvi A, Sahay A, Shear PK, Shockley KD, et al. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp Brain Res. 2005;168(3):357–67. https://doi.org/10.1007/s00221-005-0094-y.
Schmitz-Hübsch T. International cooperative ataxia rating scale (ICARS): Encyclopedia of Movement Disorders; 2010. p. 75–81.
Zhou J, Manor B, Liu D, Hu K, Zhang J, Fang J. The complexity of standing postural control in older adults: a modified detrended fluctuation analysis based upon the empirical mode decomposition algorithm. PLoS One. 2013;8(5):e62585. https://doi.org/10.1371/journal.pone.0062585.
Mancini M, Rocchi L, Horak FB, Chiari L. Effects of Parkinson’s disease and levodopa on functional limits of stability. Clin Biomech (Bristol, Avon). 2008;23(4):450–8. https://doi.org/10.1016/j.clinbiomech.2007.11.007.
Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–9. https://doi.org/10.1001/jama.291.4.460.
Melzer I, Kurz I, Oddsson LI. A retrospective analysis of balance control parameters in elderly fallers and non-fallers. Clin Biomech (Bristol, Avon). 2010;25(10):984–8. https://doi.org/10.1016/j.clinbiomech.2010.07.007.
Berry-Kravis E, Lewin F, Wuu J, Leehey M, Hagerman R, Hagerman P, et al. Tremor and ataxia in fragile X premutation carriers: blinded videotape study. Ann Neurol. 2003;53:616–23.
Wang Z, Hallac RR, Conroy KC, White SP, Kane AA, Collinsworth AL, et al. Postural orientation and equilibrium processes associated with increased postural sway in autism spectrum disorder (ASD). J Neurodev Disord. 2016;8:43. https://doi.org/10.1186/s11689-016-9178-1.
Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of DNA nucleotides. Phys Rev E. 1994;49(2):1685–9. https://doi.org/10.1103/PhysRevE.49.1685.
Jacquemont S, Leehey MA, Hagerman RJ, Beckett LA, Hagerman PJ. Size bias of fragile X premutation alleles in late-onset movement disorders. J Med Genet. 2006;43(10):804–9. https://doi.org/10.1136/jmg.2006.042374.
Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, et al. Neuronal intranucelar inclusion in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–71.
Kilby MC, Slobounov SM, Newell KM. Postural instability detection: aging and the complexity of spatial-temporal distributional patterns for virtually contacting the stability boundary in human stance. PLoS One. 2014b;9(10):e108905. https://doi.org/10.1371/journal.pone.0108905.
Kuznetsov NA, Rhea CK. Power considerations for the application of detrended fluctuation analysis in gait variability studies. PLoS One. 2017;12(3):e0174144. https://doi.org/10.1371/journal.pone.0174144.
Schmitz-Hubsch T, Tezenas du Montcel S, Baliko L, Boesch S, Bonato S, Fancellu R, et al. Reliability and validity of the International Cooperative Ataxia Rating Scale: a study in 156 spinocerebellar ataxia patients. Mov Disord. 2006;21(5):699–704. https://doi.org/10.1002/mds.20781.
Hocking DR, Birch RC, Bui QM, Menant JC, Lord SR, Georgiou-Karistianis N, et al. Cerebellar volume mediates the relationship between FMR1 mRNA levels and voluntary step initiation in males with the premutation. Neurobiol Aging. 2017;50:5–12. https://doi.org/10.1016/j.neurobiolaging.2016.10.017.
Hagerman RJ, Leavitt BR, Farzin F, Jacquemont S, Greco CM, Brunberg JA, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74(5):1051–6. https://doi.org/10.1086/420700.
Wang Z, Kwon MH, Mohanty S, Schmitt LM, White SP, Christou EA, et al. Increased force variability is associated with altered modulation of the motorneuron pool activity in autism spectrum disorder (ASD). Int J Mol Sci. 2017b;18:698. https://doi.org/10.3390/ijms18040698.
Winter DA. Human balance and posture control during standing and walking. Gait Posture. 1995;3(4):193–214.
Wang Z, Newell KM. Inter-foot coordination dynamics of quiet standing postures. Neurosci Biobehav Rev. 2014;47:194–202. https://doi.org/10.1016/j.neubiorev.2014.08.007.
Allen EG, Juncos J, Letz R, Rusin M, Hamilton D, Novak G, et al. Detection of early FXTAS motor symptoms using the CATSYS computerised neuromotor test battery. J Med Genet. 2008;45(5):290–7. https://doi.org/10.1136/jmg.2007.054676.
Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Supplement 2):ii7–ii11. https://doi.org/10.1093/ageing/afl077.
Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture. 2012;36(3):471–6. https://doi.org/10.1016/j.gaitpost.2012.04.010.
Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6(4):297–311. https://doi.org/10.1038/nrn1646.
Rosenblum M, Firsov GI, Kuuz R, Pompe B. Human postural control: force plate experiments and modelling. In: Kantz H, Kurths J, Mayer-Kress G, editors. Nonlinear analysis of physiological data. Berlin: Springer; 1998. p. 283–306.
Tassone F, Hagerman R, Chamberlain WD, Hagerman P. Transcription of the FMR1 gene in individuals with fragile x syndrome. Am J Med Genet. 2000;97:195–203.
Wang H, Ku L, Osterhout DJ, Li W, Ahmadian A, Liang Z, et al. Developmentally-programmed FMRP expression in oligodendrocytes: a potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum Mol Genet. 2004;13(1):79–89. https://doi.org/10.1093/hmg/ddh009.
Cohen S, Masyn K, Adams J, Hessl D, Rivera S, Tassone F, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67(8):1426–31. https://doi.org/10.1212/01.wnl.0000239837.57475.3a.
Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, et al. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010;30(33):10977–84. https://doi.org/10.1523/JNEUROSCI.1077-10.2010.
Leehey MA, Berry-Kravis E, Goetz CG, Zhang L, Hall DA, Li L, et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70(16 Pt 2):1397–402. https://doi.org/10.1212/01.wnl.0000281692.98200.f5.
Kraan CM, Hocking DR, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, Fielding J, et al. Cognitive-motor interference during postural control indicates at-risk cerebellar profiles in females with the FMR1 premutation. Behav Brain Res. 2013;253:329–36. https://doi.org/10.1016/j.bbr.2013.07.033.
Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, Sweeney JA. Feedforward and feedback motor control abnormalities implicate cerebellar dysfunctions in autism spectrum disorder. J Neurosci. 2015;35(5):2015–25. https://doi.org/10.1523/JNEUROSCI.2731-14.2015.
Roid GH. Stanford-Binet Intelligence Scales. Fifth ed. Itasca: Technical Manual. Riverside Publishing; 2003.
Wang JY, Hessl D, Hagerman RJ, Simon TJ, Tassone F, Ferrer E, et al. Abnormal trajectories in cerebellum and brainstem volumes in carriers of the fragile X premutation. Neurobiol Aging. 2017a;55:11–9. https://doi.org/10.1016/j.neurobiolaging.2017.03.018.
Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49(2):M72–84.
Acknowledgements
Not applicable.
Funding
This study is funded by NIMH R01 Research Project Grant Program (MH 112734), Once Upon a Time Foundation Award, the Kansas Center for Autism Research and Training (K-CART) Research Investment Council Strategic Initiative Grant to Dr. Mosconi, and the NICHD U54 Kansas Intellectual and Developmental Disabilities Research Center Award (U54HD090216).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the last author on reasonable request.
Author information
Authors and Affiliations
Contributions
MWM and ZW are responsible for the conception and design of the study. PK performed the ICARS and diagnostic evaluation for the FMR1 premutation carriers. SL provided the radiological evaluations of the T2 scan images for premutation carriers. ZW collected the behavioral data and wrote the Matlab scoring scripts for the data analyses. ZW scored the raw data and performed the statistical analyses. ZW, LMS, and MWM interpreted the results. ZW prepared the figures and drafted the manuscript. MWM, LMS, SL, and PK edited the manuscript. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All study procedures were approved by the Institutional Review Boards at the UT Southwestern Medical Center and Children’s Hospital of Dallas. Written consent was obtained from each adult individual before the administration of test and evaluations.
Consent for publication
Not applicable.
Competing interests
Dr. Wang serves as a co-investigator on an investigator-initiated award studying Phelan-McDermid Syndrome from Novartis. Dr. Mosconi serves as a consultant on this award. The other authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Correlation coefficients (r) between CGG repeat length and all COP measures and ICARS subscale and total scores of FMR1 premutation carriers without FXTAS (FXTAS− subgroup, N = 7). (DOCX 15 kb)
Additional file 2:
Table S2. Correlation coefficients (r) between CGG repeat length and all COP measures and ICARS subscale and total scores of FMR1 premutation carriers with FXTAS (FXTAS+ subgroup, N = 7). (DOCX 15 kb)
Appendix
Appendix
Detrended fluctuation analysis (DFA) is a non-linear measurement quantifying the pattern of variation of a time series across multiple time scales [17, 20]. DFA is based on the assumption that variations present in a system due to its intrinsic dynamics exhibit fractal properties of long-range correlations. To calculate the α exponent of DFA, we first let Xt represent a COP time series and then divided it into multiple non-overlapping time windows of equal length including n samples. For each time window, a local linear trend was calculated by minimizing the root mean square errors within the window and calculating Yt as a series of straight lines fits for all windows of the COP time series (N/n; N stands for the total number of samples in a trial, which is 30 sec × 200 data points/ sec = 6000 data points). The root mean square deviation from Yt (i.e., the fluctuation, F (n)) then was calculated using the following formula [26]:
This procedure was repeated over a range of different window sizes (n), and a log10-log10 coordinate of n against F (n) was constructed. A straight line on this log10-log10 graph indicates statistical self-affinity expressed as F(n) ∝ nα. The scaling exponent α is then calculated as the slope of a straight line fit to the log10-log10 graph of n against F (n) using least root mean square errors. In the current study, logarithmically spaced intervals of lengths n from 4 to 2000 samples were used and the slope α of the log10–log10 representation of F (n) versus n was estimated to fall between n = 0.02 sec and n = 10 sec. The α exponent varies between 0 and 2 (i.e., 0< α < 2). When α <0.5 or 1< α<1.5, the time series is anti-correlated with a smaller α representing increased anti-correlation and complexity. When 0.5 < α<1 or 1.5< α< 2, the signal is consistent with a greater α value representing increased long-range correlation and reduced complexity of the signal.
Glossary
- Force
-
Force is a push or a pull on an object. If the net force on an object is not zero, then the object accelerates (or changes its velocity). Force is a vector and has both magnitude and direction. Force recorded from a force platform includes measurements in three dimensions, including anterior-posterior, mediolateral, and vertical directions. Force along the vertical direction is typically referred to as the ground reaction force.
- Moment
-
The moment is the turning effect produced by a net force perpendicular to the point of rotation.
- COP
-
The point location of the vertical ground reaction force vector. The COP represents a weighted average of pressures over the surface area (i.e., feet) in contact with the ground. It has been used as an indirect measure of individuals’ postural sway. The COP can be derived from the force and moment data collected from a force platform.
- COPAP
-
Center of pressure time series in the anterior-posterior direction.
- COPML
-
Center of pressure time series in the mediolateral direction.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, Z., Khemani, P., Schmitt, L.M. et al. Static and dynamic postural control deficits in aging fragile X mental retardation 1 (FMR1) gene premutation carriers. J Neurodevelop Disord 11, 2 (2019). https://doi.org/10.1186/s11689-018-9261-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11689-018-9261-x