Abstract
The paper describes biogenic synthesis of silver nanoparticles (AgNPs) using Adhatoda vasica leaf extracts at room temperature. The prepared AgNPs were characterized by UV–visible spectroscopy, Fourier-transform infrared spectroscopy, powder X-ray diffraction, Energy dispersive X-ray (EDX), High Resolution Transmission Electron Microscope, Scanning Electron Microscopy and Thermogravimetric analyser. The bio reduction method is devoid of any toxic chemicals, organic solvents, and external reducing, capping and stabilizing agent. The synthesized AgNPs had spherical shape with particle size ranging between 3.88 and 23.97 nm and had face centered cubic structure. UV–visible spectral analysis confirmed the formation of AgNPs with a characteristic surface plasmon resonance band at 419 nm. The EDX pattern revealed the presence of elemental Ag in AgNPs. The prepared AgNPs were used for degradation of Amaranth, Allura red and Fast green in aqueous medium, with ≥ 92.6% efficiency within 15 min using 5 mg of AgNPs. The optical bandgap, Eg value of 2.26 eV for AgNPs was found to be effective for rapid photocatalytic degradation of all the three dyes. The degradation process was observed to follow pseudo first order kinetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyes industries are one such industrial sector that consumes large amounts of water and are responsible for the discharge of untreated effluents containing non-biodegradable dyes. Of approximately 1.0 million tons of dye produced annually, nearly 15% is lost in effluents from dyeing houses, causing serious environmental concerns due to their toxicity, carcinogenicity, and mutagenicity [1]. They are responsible for reduction in dissolved oxygen content, decrease in sunlight penetration in water bodies and formation of toxic substances on account of chemical or biological degradation pathways [2, 3]. Several approaches have been considered for the degradation of dyes, which includes physical, chemical, biological and radiation treatment. All these methodologies have their own advantages and limitations with several factors affecting their efficiency [4]. Moreover, some of these approaches generate secondary pollutants and thus there is a need to have alternative approaches that are more effective and economical to counter this perennial concern [4,5,6].
In the past decades, there has been a growing recognition of the potential of nanoparticles (NPs) in removing dyes from wastewater. This is due to their large surface area, strong adsorption capabilities, efficient diffusion, and quick attainment of equilibrium [7]. Although there are different physico-chemical methods like electro-spraying, laser pyrolysis, laser ablation and evaporation–condensation, combustion, thermal decomposition, sol–gel method, hydrothermal, ultrasonication, microwave-assisted combustion, and chemical reduction to prepare NPs [8,9,10], however, they require expensive experimental setup, consume higher energy, some involve use of toxic and hazardous chemicals, which are detrimental to the environment. Thus, green synthesis is widely applied to get large-scale yield of NPs, through cost effective and eco-friendly approach [11]. The biological entities like algae, bacteria, fungi and plant materials having an excellent reducing capacity, as they reduce the metal ion in zero valent state [12]. Different parts of plants such as fruit, flower, leaf, bark, seed and roots are very useful in green synthesis, as they have phytochemicals like flavonoids, phenols, terpenoids, sugar, ketones, aldehyde, carboxylic acid and amines that act as reducing as well as stabilizing agent [13].
Many metal-based NPs have been synthesised via green approach, but AgNPs have attracted significant attention as they possess excellent physical, chemical, and biological properties, making them suitable for a wide range of applications. This includes different fields like pharmaceuticals, electronics, biomedicine, drug delivery, biomedical devices, delivery of peptides, environmental monitoring, dye degradation, and in control of infectious diseases [7, 14, 15]. They also show anticancer, antifungal, antibacterial, antiprotozoal, and antimicrobial activity against gram-positive and gram-negative bacteria [13]. Raveendran and co-workers [16] were the first to report the synthesis of AgNPs using a green method. The process was simple and eco-friendly, wherein the solution of silver nitrate and β-d glucose was heated with an aqueous solution of starch to obtain monodispersed AgNPs. In their method, β-d glucose acted as a reducing agent while starch was used as a stabilizing agent. Since then, many researchers have prepared AgNPs using different plant materials like, Diospyros lotus (leaf) [17], Berberis vulgaris (leaf and root) [18], Thymus kotschyanus [19], Sapota (Fruit) [20], Tulsi (leaf) [21], Zanthoxylum armatum (leaf) [3], Mussaendraerythrophylla (leaf) [22], Artemisia annua (leaf) [23], Saraca indica (flower) [24], Trapa bispinosa (peel) [25], Garlic [26], Vitex negundo (Leaf) [27], Medicago sativa (seed) [28], Nicotiana tobaccum (leaf) [29], Sorghum bicolor (bran) [30], Allium cepa [31], Azadirachta indica (leaf) [32], Gliricidiasepium (leaf) [33], Euphorbia hirta (leaf) [34], Boswellia Ovalifoliolata (bark) [35], Jatropha curcas (latex) [36], Carica papaya (fruit) [37], Cinnamomum camphora (leaf) [38], Capsicum annuum [39], Emblica officinalis (fruit) [40], Aloevera [41], Pelargonium graveolens (leaf) [42], Dalbergia sisoo (leaf) [43] and Angelica gigas ribbed stem extracts [44]. Additionally, few other methods have reported the use of Annona muricata L. and Acmella uliginosa leaf extract to prepare efficient catalysts for photocatalytic degradation of malachite green dye [45, 46].
According to Ayurveda and Unani medicine, Adhatoda vasica is a very useful medicinal plant, and is used as an indigenous medicine for many centuries [47, 48]. It belongs to Acanthaceae family and contains alkaloids, triterpenoid, flavonoid, chalcone, steroid, alkyl ketones, alkyl hydroxyl ketone, glucoside, amino acid, and organic acids. These compounds are responsible for reduction and stabilization of metal ions [47]. Bhumi and co-workers synthesized AgNPs from Adhatoda vasica leaf extract and demonstrated their antifungal and antibacterial activity against S. aureus, Pseudomonas aeuroginosa, E. coli, B. thuringiensis and K. Pneumonia [49]. Catalytic degradation of methylene blue and methylene green dyes with AgNPs prepared from Adhatoda vasica leaf extract has also been reported [50]. They also investigated the antioxidant, antialgal and antifungal potential of green synthesized AgNPs. Similarly, another study reported the degradation of orange and blue dyes using AgNPs synthesized from Adhatoda vasica leaf extract [51]. Antibacterial activity of these nanoparticles has been studied against Pseudomonas aeruginosa MTCC 741 [52] and against gram-positive and gram-negative pathogenic bacterium [53]. Karthick et al. [54] and Bharathi et al. [55] described the green synthesis of AuNPs using Adhatoda vasica Nees, however, no application was discussed.
The aim of this work was to develop a simple, green, and cost-effective method to prepare AgNPs and study their physicochemical properties. In the present work we investigated the leaf extract of Adhatoda vasica which acted as the stabilizer and also as a reducing agent to prepare AgNPs. To the best of our knowledge, this is the first report for its application in the photocatalytic degradation of Amaranth, Allura red and Fast green dyes which are widely used in food, beverage, and cosmetic industries. One notable aspect of the work was its ability to achieve the rapid degradation of these dyes in just 60 min, showcasing remarkable efficiency. The synthesized AgNPs were characterized using different analytical tools such as UV Visible spectroscopy, Fourier Transformer Infrared (FTIR) Spectroscopy; High Resolution Transmission Electron Microscopy (HRTEM); X-ray Diffraction (XRD) analysis, Selected Area Electron diffraction (SAED) pattern, Scanning Electron Microscopy (SEM)and Energy Dispersive X-ray (EDX) and Thermogravimetric analyser (TGA). Furthermore, the degradation kinetics of these dyes is also presented.
Materials and method
Plant and chemicals
The leaves of Adhatoda vasica were obtained from Ahmedabad district, Gujarat, India (N 23°2′18.3768; E72°32′34.8468) during the summer season of 2022. The sample leaves were identified and authenticated by the Department of Botany, Gujarat University, Gujarat, India. The experimental research carried out using this plant, including the collection of plant material, complied with the Gujarat University guidelines and legislation. Silver nitrate (AgNO3) was purchased from Finar Limited, Gujarat. Allura red (E129), Amaranth (E123) and Fast green (E143) dyes were procured from Sigma Aldrich (Germany). Further, all other chemicals used were of analytical grade.
Preparation of aqueous leaf extract of Adhatoda vasica
The fresh and undamaged leaves of Adhatoda vasica were first cleaned thoroughly using tap water and then washed carefully with Milli Q water. Thereafter the leaves were shade dried and 15 g of these dried leaves were chopped into small pieces and placed in a glass beaker. To this, 100 mL of Milli Q water was added, and the mixture was heated at 65 °C for 45 min. Subsequently, the solution was filtered using a Whatman filter paper no. 41, which had a pore size of 20–25 µm. The resulting leaf extract had a pale-yellow color and was used to reduce, cap, and stabilize the AgNPs. Finally, the extract was stored in a refrigerator at a temperature of 2 °C away from direct light and to prevent any agglomeration.
Preparation of AgNPs using Adhatoda vasica leaf extract
A solution of 0.05 M AgNO3 was prepared using Milli Q water and 50 mL portion of the solution was taken in a conical flask. Since AgNO3 is sensitive to light and can convert into silver oxide in the presence of sunlight or bright light, the conical flask containing the AgNO3 solution was covered with aluminum foil. Next, the leaf extract was added dropwise to the AgNO3 solution under constant stirring at 480 rpm on a magnetic stirrer. After adding 7 mL of the extract, the color of AgNO3 solution changed from colorless to brown, indicating the formation of AgNPs. To purify the solution containing the green-synthesized AgNPs, it was centrifuged at 6000 rpm for 20 min until a clear supernatant was obtained. The supernatant was removed, and the resulting pellet was washed thrice with Milli Q water. The pellet was then dried overnight at room temperature and used for characterization and further analysis.
Characterization of AgNPs
The prepared AgNPs were characterized by UV–visible spectrometer (JASCO V-630) in range of 200–800 nm. The absorption data was also used to find the band gap of AgNPs. To identify the functional groups, present in leaf extract, which are responsible for reducing, capping, and stabilizing action, FTIR (Agilent Micro lab) spectral analysis was performed. Using the powder form of AgNPs the FTIR spectrum was recorded from 500 to 4000 cm−1. XRD (Rigaku Smart Lab 2) was used to study the phase and crystalline structure of AgNPs. The XRD instrument was utilized with a 40 kV voltage and a 45 mA electric current, using Cu K-alpha radiation as the X-ray source. The diffractogram was recorded between 20 and 80 (2θ) angles. The Debye Scherrer formula was employed to determine the mean crystallite size of AgNPs, while qualitative and quantitative analysis of the elemental composition of AgNPs synthesized through the green method was conducted using EDX (Carl Zeiss Supra 55). Information regarding size, shape and distribution of particles were carried out using HRTEM (JEM-2100 high-resolution analytical TEM), operated at a voltage of 200 kV. TGA was employed to investigate the decomposition behaviour and thermal properties of AgNPs. The thermal analysis was conducted using a NETZSCH STA 2500 instrument in a nitrogen atmosphere, with temperature ranging from 28 to 1050 °C and a heating rate of 10 °C per min.
Photocatalytic degradation of dye
The study aimed to investigate the photocatalytic degradation of Amaranth, Allura red, and Fast green dyes under sunlight using AgNPs synthesized through a green method. To prepare stock dye solutions, a concentration of 1 × 10–3 M was achieved by dissolving each dye in Milli-Q water. Four beakers were filled with 100 mL of the stock dye solution of Amaranth dye. In three of the beakers, 5 mg, 10 mg, and 15 mg of green synthesized AgNPs were added and thoroughly mixed. The fourth beaker served as a reference solution and did not have AgNPs. Subsequently, all four beakers were exposed to sunlight during the summer season, with an atmospheric temperature of 38 °C. The same procedure was repeated for Allura red and Fast green dyes. The UV–visible spectrophotometer was employed to measure the absorbance of all the dyes within the wavelength range of 300–800 nm. The maximum absorbance values were observed at 521 nm, 500 nm, and 624 nm for Amaranth, Allura red, and Fast green, respectively.
The percentage of dye degradation was found using the expression, % of degradation = 1-\(\frac{At}{{A0}}\) × 100″, where \(A_{0}\) is the initial absorbance of dye and \(A_{t}\) is the absorbance of dye at time ‘t’. To study the kinetics of dye degradation reaction, the values of ln [C] vs t (1st order), 1/C vs t (2nd order), ln \(\left( {\frac{C0}{{Ct}}} \right)\) vs t (Pseudo 1st order) were plotted and the coefficient of determination (\(R^{2}\)) for each graph was estimated to find the order of reaction.
Results and discussion
Process optimization for the synthesis of AgNPs using Adhatoda vasica
To prepare stable AgNPs, different parameters such as pH (3–9), volume of plant extract (5–15 mL), AgNO3 concentration (0.01–0.1 M), reaction temperature (25–45 °C) and reaction time (5–20 min) were studied. The optimum reaction conditions found were, pH 3; volume of extract, 7 mL; 50 mL, 0.05 M AgNO3; 25 °C temperature and 10 min of reaction time. The major constituents of Adhatoda vasica leaf extract includes pyrroloquinazoline alkaloids, vasicine, vasicol, adhatonine, vasicinone, vasicinol, vasicinolone, besides different glycosides, saponins, flavonoids and other phenolic compounds [54, 55]. The phytochemicals mainly responsible for the reduction of silver ions to AgNPs in solution are the phenols and alkaloids. The chemical reactions that are involved in converting silver ions into AgNPs are as follows,
The hydrated electrons (eaq−) act a strong reducing agent and thus reduce Ag+ ions into zero-valent Ag atoms (Ago) (Eq. 2). The solution containing Ago was centrifuged and after decantation of the supernatant, AgNPs pellet was obtained. Additionally, flavonoids, tannins, and saponins serve as natural surfactants and capping agents in the formation of nanoparticles and prevent agglomeration [45, 46].
UV–Visible spectroscopy
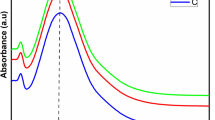
The prepared AgNPs showed an absorption peak at 419 nm (Fig. 1), which in accordance with a previous report, where AgNPs exhibits maximum absorption between 410 and 430 nm [56]. The leaf extract had an absorption maximum at 345 nm. The colour of green synthesized AgNPs remained stable for 3 weeks at 2 °C with no indication of oxidation, aggregation, and precipitation. This test was carried out in triplicate. However, there was a slight decrease in the absorbance with no change in the wavelength maxima, \(\lambda_{max}\) after 3 weeks as shown in Fig. 3. This suggests that green synthesized AgNPs had high stability due to the presence of stabilizing and capping agents in the leaf extract. The UV–visible spectra were further used to find the optical band gap of AgNPs using Tauc plot, which is expressed as (αhν)2 = (hν-Eg), where α represents the absorptivity co-efficient and hν is the optical energy of the system [57]. The plot of (αhν)2 against hν and a tangent of curve extrapolated on X-axis gave the value of band gap (Eg) of AgNPs, as shown in Fig. 2. The Eg value of AgNPs obtained was 2.26 eV. Nanoparticles with a smaller Eg value are highly beneficial in various applications. Additionally, this characteristic allows for easier generation of electrons and holes, making these AgNPs more receptive to low-energy electromagnetic radiation, resulting in more efficient photocatalytic dye degradation [56].
Fourier-transform infrared spectroscopy
The prepared AgNPs were analysed by FTIR spectroscopy to identify functional groups present in the biomolecules that assisted in the reduction, capping and stabilization of the NPs. These functional groups are responsible for bioreduction of silver ion (Ag+) into zero valent silver atom (Ag0). Figure 3 shows the FTIR spectra of Adhatoda vasica leaf extract and the synthesized AgNPs. The major vibrational peaks observed in leaf extract were, 3335.76 cm−1 due to stretching vibration of –OH (hydroxyl group), 2918.50 cm−1 due to C-H stretch (aliphatic), 2851.41 cm−1 on account of stretching vibration of –NH (amine group), 1636.30 cm−1 due to stretching vibration of C=O(carbonyl group), 1416.38 cm−1 can be ascribed to C=C bond (aromatic) stretching, 1241.20 cm−1 can be related to stretching vibration of –C–O– (aromatic ester) and 1025.01 due to stretching vibration of –COO– (anhydride group). The O–H stretching vibration can be attributed to the O–H bond of flavonoids, saponins, and tannins. On the other hand, the C=C and C–H stretching vibrations indicate the presence of alkaloids, saponins, flavonoids, and tannins in Adhatoda vasica [45, 46]. According to the FTIR spectrum of the green synthesized AgNPs the peaks observed at 3272.60 cm−1, 2918.50 cm−1, 2851.41 cm−1, 1543.11 cm−1, 1367.93 cm−1, 1241.20 cm−1, and 1025.01 cm−1, were identical to the peaks that appeared in the FTIR spectrum of the leaf extract but the peak intensity was comparatively less compared to the leaf extract. However, some of the peaks of Adhatoda vasica leaf extract appeared to have shifted in the synthesized AgNPs, as follows, 3335.76 cm−1 to 3272.60 cm−1, 1636.30 cm−1 to 1543.11 cm−1 and 1416.38 cm−1 to 1367.93 cm−1. The results obtained confirmed the presence of phytochemicals in Adhatoda vasica leaf extract, which could have acted as reducing and capping agents for AgNPs.
XRD analysis
XRD analysis was employed to ascertain the crystalline composition of AgNPs. XRD analysis was used to identify the crystalline phase of AgNPs. The XRD spectrum of synthesized AgNPs is shown in Fig. 4. The presence of significant and acute diffraction peaks at 27.77°, 29.51°, 32.24°, 38.11°, 46.25°, 54.88°, 57.62°, and 77.21orelated to (3 0 1), (4 0 0), (0 0 2), (5 1 0), (3 3 2), (4 4 2), (3 2 3), and (3 3 4) planes, respectively, suggests crystalline face centered cubic (FCC) phase [58]. The diffraction patterns of Ag can be correlated with the reference data found in the Joint Committee on Powder Diffraction Standards (JCPDS) files, specifically 96–900–8640 and 96–901-1667. Thus, from the observed pattern we can conclude face centered cubic structure of AgNPs (a = b = c = 3.9165 and α = β = ϒ 90°) [59].
Particle size plays a crucial role in the green synthesis of nanoparticles, and Scherer's formula is employed to calculate nanoparticle size using the full width at half maximum (FWHM) position [60].
where τ signifies the crystal thickness, measured perpendicular to the reflecting plane, k is the Scherrer’s constant (for spherical particles the value is 0.9), wavelength (λ) of the X-ray radiation is 1.5405 Å, β2θ (in radians) represents the width at half the maximum intensity, and θ is the Bragg’s angle [58]. The calculated crystallite range varied between 8 and 18 nm depending on the type of reflection used for the AgNPs. The average crystalline size of prepared AgNPs was 10.84 nm, which agrees with HRTEM result. The calculated data of lattice strain and dislocation density were found from the data using customized Scherrer formula as shown in Table 1 [61].
HRTEM, EDAX and SEM analysis
The particle size and morphology of the synthesized AgNPs were determined by HRTEM analysis. According to the HRTEM micrograph (Fig. 5a), the size of AgNPs ranged between 3.88 and 23.97 nm and average particles size was 11.36 nm. The particles were spherical in shape and their size distribution is represented by a histogram (Fig. 5b). SAED pattern analysis gave an idea regarding crystalline nature of the prepared AgNPs. It showed diffraction rings with intermittent dots, suggesting crystalline nature of AgNPs (Fig. 5c). These concentric rings correspond to diffraction planes present in AgNPs and were indexed as (510), (332), (442), (323), and (334), which agrees with the XRD data.
The elemental composition of green synthesized silver nanoparticles was assessed by EDX spectroscopy. A strong signal for silver [Ag (0), At. (49.6%)] was observed at 3 keV, which confirmed the presence of AgNPs (Fig. 6). The presence of carbon (At. 24.1%) and oxygen (At. 16.8%) in the EDX spectra can be attributed to the presence of capping and stabilizing agents present on the surface of AgNPs. The spectra also showed the presence of chlorine (At. 9%) as reported previously [55, 62]. The SEM micrograph showed spherical morphology of the AgNPs with the average size below 20 nm as presented in Fig. 7. The result further supports the size of particles estimated by XRD analysis.
TGA analysis
Thermal stability of green synthesized AgNPs and mass loss of AgNPs were determined by TGA analysis. TGA spectra shown in Fig. 8 displayed first mass loss of 9.26% between 96 and 220 °C due to the removal of water molecules present on the surface of AgNPs. The second mass loss (23.08%) observed up to 625 °C, was mainly due to the evaporation and decomposition of different phytochemicals present on surface of AgNPs, which serve as a capping and stabilizing agent in the green synthesis. Between 900 and 1000 °C, mass percentage becomes constant, around 60%. This shows that further mass loss was not observed due to the presence of silver metal.
Photocatalytic degradation of dye
To assess the photocatalytic activity of the prepared AgNPs, three different dyes namely Amaranth, Allura red and Fast green were studied. Initially, different amounts of AgNPs (5.0, 10.0 and 15.0 mg) were evaluated to optimize the best conditions for degradation. However, it was found that there was no significant difference in the percentage of dye degradation (89–92%). Thus, photodegradation was carried out by adding 5.0 mg AgNPs to 100 mL, 1.0 × 10–3 M aqueous solution of each dye in the presence of sunlight. It was observed that there was an immediate decrease in the absorption for all the dyes. With increase in the exposure time there was a corresponding decrease in the absorption peaks for the dyes. The UV–visible spectra in Fig. 9a–c shows time-dependent degradation of these dyes using 5.0 mg AgNPs. The absorbance measurements were made after tenfold dilution of the samples. The absorption peaks at 521 nm, 500 nm and 624 nm corresponding to Amaranth, Allura red and Fast green, respectively degraded substantially after 1 h of exposure. The results for experiment carried out using different amounts of AgNPs in the presence of sunlight is shown in Table 2. Significant degradation (in the range of 92–95%) was observed for the dyes using 5.0 mg AgNPs after 1 h exposure, however, the corresponding increase was not substantially higher with 10.0 mg or 15.0 mg AgNPs. To justify the photocatalytic activity of the prepared AgNPs, the experiment was also performed in the absence of sunlight, the results of which are represented in Table 3. Further, a control experiment was performed by keeping the dye solutions under sunlight without AgNPs, however, there was negligible degradation of the dye samples. To compare the efficiency of the prepared AgNPs for the degradation of dyes, an assessment of the results obtained from reported studies [63,64,65,66,67,68,69,70] for photocatalytic degradation of Amaranth, Allura red and Fast green is shown in Table 4.
Based on the current research, it has been determined that to treat a typical wastewater containing 15 mmoles of dyes, the photocatalytic dye degradation requires 2.8 g of AgNPs using the present method. Additionally, this amount of AgNPs can be obtained from 15 g of leaves from the Adhatoda vasica plant. These nanoparticles possess the ability to degrade the dyes within 60 min with approximately 96% efficiency. However, future advancements in this field would aim to enhance the efficiency, reduce costs, and shorten the degradation time by utilizing AgNPs-based nanocomposites, such as those synthesized using environmentally friendly techniques with activated carbon, graphene oxide, etc. Furthermore, incorporating silica spheres to support the AgNPs could enhance the catalytic efficiency of nanoscale materials. Consequently, the resulting materials would exhibit greater versatility in degrading various types of dyes.
Further, the kinetics of dye degradation showed pseudo-first order as evident from the plots of ln(C0/Ct) vs. irradiation time ‘t’ for the dyes, where Ct and Co represent the concentration of the dye at time ‘t’ and ‘0’, respectively (Fig. 10). The plots showed a linear relationship for all the dyes and the slope values corresponded to the pseudo-first order rate constant (k) for the degradation of the dyes. The value of k was found for Amaranth, Allura red and Fast green was 6.02 × 10−2, 6.19 × 10−2 and 8.43 × 10−2 min−1, respectively. These values of rate constant, k was essentially dependent on the chemical structure of the dyes. Thus, it can be concluded that the prepared AgNPs were highly efficient for the degradation of these dyes.
Conclusion
This study presents a promising approach to produce AgNPs using leaf extract of Adhatoda Vasica. The developed method eliminates the need for organic solvents, surfactants, capping agents, and templates. It highlights the dual functional ability of the Adhatoda Vasica extract solution. As a result, this fabrication method is straightforward, environmentally friendly, cost-effective, and sustainable. The synthesized AgNPs showed high efficiency to degrade three dyes namely, Amaranth, Allura Red and Fast green within 1 h. Further, it was possible to obtain 2.8 g of AgNPs using 15 g of Adhatoda vasica leaves which could efficiently degrade 15 mmoles of dyes present in water samples. This efficacy showcases their potential as efficient photocatalysts for industrial effluent treatment. Therefore, this technique holds significant promise for the fabrication of AgNPs and their application in environmental remediation.
Data availability
There is no data to report other than that presented in this study.
Code availability
Not applicable.
References
Li P, Song Y, Wang S, Tao Z, Yu S, Liu Y. Enhanced decolorization of methyl orange using zero-valent copper nanoparticles under assistance of hydrodynamic cavitation. Ultrason Sonochem. 2015;22:132–8. https://doi.org/10.1016/j.ultsonch.2014.05.025.
Nakkala JR, Bhagat E, Suchiang K, Sadras SR. Comparative study of antioxidant and catalytic activity of silver and gold nanoparticles synthesized from Costus pictus leaf extract. J Mater Sci Technol. 2015;31:986–94. https://doi.org/10.1016/j.jmst.2015.07.002.
Jyoti K, Singh A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J Genet Eng Biotechnol. 2016;14:311–7. https://doi.org/10.1016/j.jgeb.2016.09.005.
Kumar MD, Tortajada C. Assessing wastewater management in India. Singapore: Springer; 2020. p. 23–30. https://doi.org/10.1007/978-981-15-2396-0.
Jain K, Patel AS, Pardhi VP, Flora SJS. Nanotechnology in wastewater management: a new paradigm towards wastewater treatment. Molecules. 2021;26:1797. https://doi.org/10.3390/molecules26061797.
Marimuthu S, Antonisamy AJ, Malayandi S, Rajendran K, Tsai PC, Pugazhendhi A, Ponnusamy VK. Silver nanoparticles in dye effluent treatment: a review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J Photochem Photobiol B Biol. 2020;205:111823. https://doi.org/10.1016/j.jphotobiol.2020.111823.
Selvam GG, Sivakumar K. Phycosynthesis of silver nanoparticles and photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Hypnea musciformis (Wulfen) JV Lamouroux. Appl Nanosci. 2015;5:617–22. https://doi.org/10.1007/s13204-014-0356-8.
Singh TA, Das J, Sil PC. Zinc oxide nanoparticles: a comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interface Sci. 2020;286:102317. https://doi.org/10.1016/j.cis.2020.102317.
Akintelu SA, Folorunso AS, Folorunso FA, Oyebamiji AK. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon. 2020;6:e04508. https://doi.org/10.1016/j.heliyon.2020.e04508.
Khan MI, Akhtar MN, Ashraf N, Najeeb J, Munir H, Awan TI, Tahir MB, Kabli MR. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl Nanosci. 2020;10:2351–64. https://doi.org/10.1007/s13204-020-01414-x.
Kotval SC, John T, Parmar KA. Green synthesis of copper nanoparticles using Mitragyna parvifolia plant bark extract and its antimicrobial study. J Nanosci Nanotechnol. 2018;4:456–60. https://doi.org/10.30799/jnst.133.18040415.
Nagaraj E, Shanmugam P, Karuppannan K, Chinnasamy T, Venugopal S. The biosynthesis of a graphene oxide-based zinc oxide nanocomposite using Dalbergia latifolia leaf extract and its biological applications. New J Chem. 2020;44:2166–79. https://doi.org/10.1039/c9nj04961d.
Nasrollahzadeh M, Mahmoudi-GomYek S, Motahharifar N, Ghafori Gorab M. Recent developments in the plant-mediated green synthesis of Ag-based nanoparticles for environmental and catalytic applications. Chem Rec. 2019;19:2436–79. https://doi.org/10.1002/tcr.201800202.
Homaeigohar S. The nanosized dye adsorbents for water treatment. Nanomaterials. 2020;10:295. https://doi.org/10.3390/nano10020295.
Ledakowicz S, Paździor K. Recent achievements in dyes removal focused on advanced oxidation processes integrated with biological methods. Molecules. 2021;26:870. https://doi.org/10.3390/molecules26040870.
Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125:13940–1. https://doi.org/10.1021/ja029267j.
Hamedi S, Shojaosadati SA. Rapid and green synthesis of silver nanoparticles using Diospyros lotus extract: evaluation of their biological and catalytic activities. Polyhedron. 2019;171:172–80. https://doi.org/10.1016/j.poly.2019.07.010.
Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol. 2019;124:148–54. https://doi.org/10.1016/j.ijbiomac.2018.11.101.
Hamelian M, Zangeneh MM, Amisama A, Varmira K, Veisi H. Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl Organomet Chem. 2018;32:e4458. https://doi.org/10.1002/aoc.4458.
Vishwasrao C, Momin B, Ananthanarayan L. Green synthesis of silver nanoparticles using sapota fruit waste and evaluation of their antimicrobial activity. Waste Biomass Valorization. 2019;10:2353–63. https://doi.org/10.1007/s12649-018-0230-0.
Singh J, Mehta A, Rawat M, Basu S. Green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-Nitrophenol reduction. J Environ Chem Eng. 2018;6:1468–74. https://doi.org/10.1016/j.jece.2018.01.054.
Varadavenkatesan T, Selvaraj R, Vinayagam R. Phyto-synthesis of silver nanoparticles from Mussaenda erythrophylla leaf extract and their application in catalytic degradation of methyl orange dye. J Mol Liq. 2016;221:1063–70. https://doi.org/10.1016/j.molliq.2016.06.064.
Khatoon N, Ahmad R, Sardar M. Robust and fluorescent silver nanoparticles using Artemisia annua: biosynthesis, characterization and antibacterial activity. Biochem Eng J. 2015;102:91–7. https://doi.org/10.1016/j.bej.2015.02.019.
Vidhu VK, Philip D. Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flower. Spectrochim Acta A Mol Biomol Spectrosc. 2014;117:102–8. https://doi.org/10.1016/j.saa.2013.08.015.
Pandey S, Mewada A, Thakur M, Shinde S, Shah R, Oza G, Sharon M. apid biosynthesis of silver nanoparticles by exploiting the reducing potential of Trapa bispinosa peel extract. J Nanosci. 2013;2013:1–9. https://doi.org/10.1155/2013/516357.
Von White G, Kerscher P, Brown RM, Morella JD, McAllister W, Dean D, Kitchens CL. Green synthesis of robust, biocompatible silver nanoparticles using garlic extract. J Nanomater. 2012;2012:730746. https://doi.org/10.1155/2012/730746.
Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, Jahanshiri F, Farahani F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules. 2011;16:6667–76. https://doi.org/10.3390/molecules16086667.
Lukman AI, Gong B, Marjo CE, Roessner U, Harris AT. Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci. 2011;353:433–44. https://doi.org/10.1016/j.jcis.2010.09.088.
Prasad KS, Pathak D, Patel A, Dalwadi P, Prasad R, Patel P, Selvaraj K. Biogenic synthesis of silver nanoparticles using Nicotiana tobaccum leaf extract and study of their antibacterial effect. Afr J Biotechnol. 2011;10:8122–30. https://doi.org/10.5897/AJB11.394.
Njagi EC, Huang H, Stafford L, Genuino H, Galindo HM, Collins JB, Hoag GE, Suib SL. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir. 2011;27:264–71. https://doi.org/10.1021/la103190n.
Saxena A, Tripathi RM, Singh RP. Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig J Nanomater Bios. 2010;5:427–32.
Tripathy A, Raichur AM, Chandrasekaran N, Prathna TC, Mukherjee A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanopart Res. 2010;12:237–46. https://doi.org/10.1007/s11051-009-9602-5.
Raut RW, Lakkakula JR, Kolekar NS, Mendhulkar VD, Kashid SB. Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr Nanosci. 2009;5:117–22. https://doi.org/10.2174/157341309787314674.
Elumalai EK, Prasad TN, Hemachandran J, Therasa SV, Thirumalai T, David E. Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci Res. 2010;2:549–54.
Ankanna ST, Prasad TN, Elumalai EK, Savithramma N. Production of biogenic silver nanoparticles using Boswellia ovalifoliolata stem bark. Dig J Nanomater Bios. 2010;5:369–72.
Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Asp. 2009;339:134–9. https://doi.org/10.1016/j.colsurfa.2009.02.008.
Jain D, Daima HK, Kachhwaha S, Kothari SL. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig J Nanomater Bios. 2009;4:557–63.
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J. Biosynthesis of silver and gold nanoparticles by novel sundried cinnamomum camphora leaf. Nanotechnology. 2007;18:105104. https://doi.org/10.1088/0957-4484/18/10/105104.
Li S, Shen Y, Xie A, Yu X, Qiu L, Zhang L, Zhang Q. Green synthesis of silver nanoparticles using Capsicum annuum L extract. Green Chem. 2007;9:852–8. https://doi.org/10.1039/b615357g.
Ankamwar B, Damle C, Ahmad A, Sastry M. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol. 2005;5:1665–71. https://doi.org/10.1166/jnn.2005.184.
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using aloevera plant extract. Biotechnol Prog. 2006;22:577–83. https://doi.org/10.1021/bp0501423.
Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19:1627–31. https://doi.org/10.1021/bp034070w.
Deep A, Verma M, Marwaha RK, Sharma AK, Kumari B. Development, characterization and anticancer evaluation of silver nanoparticles from Dalbergia sissoo leaf extracts. Curr Cancer Ther Rev. 2019;15:1–7. https://doi.org/10.2174/1573394715666190820150651.
Chokkalingam M, Rupa EJ, Huo Y, Mathiyalagan R, Anandapadmanaban G, Ahn JC, Park JK, Lu J, Yang DC. Photocatalytic degradation of industrial dyes using Ag and Au nanoparticles synthesized from Angelica gigas ribbed stem extracts. Optik. 2019;185:1213–9. https://doi.org/10.1016/j.ijleo.2019.04.065.
Halomoan I, Yulizar Y, Surya RM, Apriandanu DOB. Facile preparation of CuO-Gd2Ti2O7 using Acmella uliginosa leaf extract for photocatalytic degradation of malachite green. Mat Res Bull. 2022;150:111726. https://doi.org/10.1016/j.materresbull.2021.111726.
Yulizar Y, Abdullah I, Surya RM, Alifa NL. Green synthesis of novel YMnO3-doped TiO2 for enhanced visible-light- driven photocatalytic degradation of malachite green. J Environ Manag. 2023;342:118139. https://doi.org/10.1016/j.jenvman.2023.118139.
Singh TP, Singh OM, Singh HB. Adhatoda vasica Nees: phytochemical and pharmacological profile, Adhatoda vasica nees: phytochemical and pharmacological profile. Nat Prod J. 2011;1:29–39. https://doi.org/10.2174/2210316311101010029.
Bhavyasree PG, Xavier TS. Green synthesis of copper oxide/carbon nanocomposites using the leaf extract of Adhatoda vasica Nees, their characterization and antimicrobial activity. Heliyon. 2020;6:e03323. https://doi.org/10.1016/j.heliyon.2020.e03323.
Bhumi G, Rao ML, Savithramma N. Green synthesis of silver nanoparticles from the leaf extract of Adhatoda vasica nees. and assessment of its antibacterial activity. Asian J Pharm Clin Res. 2015;8:62–7.
Bibi B, Tahir A, Arshad M, Khan S, Anum F, Bashir F, Raza S. Green synthesis of silver nanoparticles by Adhatoda vasica L. Pure Appl Biol. 2020;9(4):2415–24. https://doi.org/10.19045/bspab.2020.90256.
Gola D, Kriti A, Bhatt N, Bajpai M, Singh A, Arya A, Chauhan N, Srivastava SK, Tyagi PK, Agrawal Y. Silver nanoparticles for enhanced dye degradation. Curr Res Green Sustain Chem. 2021;4:100132. https://doi.org/10.1016/j.crgsc.2021.100132.
Bose D, Chatterjee S. Antibacterial activity of green synthesized silver nanoparticles using Vasaka (Justicia adhatoda L.) leaf extract. Indian J Microbiol. 2015;55:163–7. https://doi.org/10.1007/s12088-015-0512-1.
Nazeruddin GM, Prasad NR, Prasad SR, Garadkar KM, Nayak AK. In-vitro bio-fabrication of silver nanoparticle using Adhathoda vasica leaf extract and its anti-microbial activity. Phys E. 2014;61:56–61. https://doi.org/10.1016/j.physe.2014.02.023.
Karthick V, Kumar VG, Maiyalagan T, Deepa R, Govindaraju K, Rajeswari A, Stalin Dhas T. Green synthesis of well dispersed nanoparticles using leaf extract of medicinally useful Adhatoda vasica nees. Micro Nanosyst. 2012;4:192–8. https://doi.org/10.2174/1876402911204030192.
Bharathi D, Ramalakshmi S, Kalaichelven PT. Akilakalaichelvan, Biological synthesis of silver nanoparticles by using leaf extract of Justicia adhatoda. Der Pharm Lett. 2015;7(7):391–5.
Munir H, Mumtaz A, Rashid R, Najeeb J, Zubair MT, Munir S, Bilal M, Cheng H. Eucalyptus camaldulensis gum as a green matrix to fabrication of zinc and silver nanoparticles: Characterization and novel prospects as antimicrobial and dye-degrading agents. J Mater Res Technol. 2020;9:15513–24. https://doi.org/10.1016/j.jmrt.2020.11.026.
Dodoo-Arhin D, Asiedu T, Agyei-Tuffour B, Nyankson E, Obada D, Mwabora JM. Photocatalytic degradation of Rhodamine dyes using zinc oxide nanoparticles. Mater Today Proc. 2021;38:809–15. https://doi.org/10.1016/j.matpr.2020.04.597.
Elton LR, Jackson DF. X-ray diffraction and the Bragg law. Am J Phys. 1966;34:1036–8. https://doi.org/10.1119/1.1972439.
Scheel HJ, Fukuda T. The development of crystal growth technology. In: Crystal growth technology. New York: Wiley; 2003. https://doi.org/10.1002/0470871687.
Mott DM, Mai NT, Thuy NT, Sakata T, Higashimine K, Koyano M, Maenosono S. Elucidation of the complex structure of nanoparticles composed of bismuth, antimony, and tellurium using scanning transmission electron microscopy. J Phys Chem C. 2011;115:17334–40. https://doi.org/10.1021/jp205588e.
Arsenlis A, Parks DM. Modeling the evolution of crystallographic dislocation density in crystal plasticity. J Mech Phys Solids. 2002;50:1979–2009. https://doi.org/10.1016/S0022-5096(01)00134-X.
Ahmed T, Noman M, Shahid M, Niazi MBK, Hussain S, Manzoor N, Wang X, Li B. Green synthesis of silver nanoparticles transformed synthetic textile dye into less toxic intermediate molecules through LC-MS analysis and treated the actual wastewater. Environ Res. 2020;191:110142. https://doi.org/10.1016/j.envres.2020.110142.
Lin KYA, Lin JT, Lu XY, Hung C, Lin YF. Electrospun magnetic cobalt-embedded carbon nanofiber as a heterogeneous catalyst for activation of oxone for degradation of Amaranth dye. J Colloid Interface Sci. 2017;505:728–35. https://doi.org/10.1016/j.jcis.2017.06.057.
Steter JR, Barros WRP, Lanza MRV, Motheo AJ. Electrochemical and sonoelectrochemical processes applied to amaranth dye degradation. Chemosphere. 2014;117:200–7. https://doi.org/10.1016/j.chemosphere.2014.06.085.
Alwash AH, Abdullah AZ, Ismail N. Zeolite Y encapsulated with Fe-TiO2 for ultrasound-assisted degradation of amaranth dye in water. J Hazard Mater. 2012;233–234:184–93. https://doi.org/10.1016/j.jhazmat.2012.07.021.
Anand K, Kaviyarasu K, Muniyasamy S, Roopan SM, Gengan RM, Chuturgoon A. Bio-synthesis of silver nanoparticles using agroforestry residue and their catalytic degradation for sustainable waste management. J Clust Sci. 2017;28:2279–91. https://doi.org/10.1007/s10876-017-1212-2.
Salem MA, Al-Ghonemiy AF, Zaki AB. Photocatalytic degradation of Allura red and quinoline yellow with polyaniline/TiO2 nanocomposite. App Catal B Environm. 2009;91:59–66. https://doi.org/10.1016/j.apcatb.2009.05.027.
Sanjurjo-García JA, Martínez-Gallegos S, Schabes-Retchkiman PS, García-Rivas JL. Degradation of allura red dye using Fe-Zn metal nanoparticles obtained by phytosynthesis method. MRS Adv. 2020;5:3283–91. https://doi.org/10.1557/adv.2020.392.
Sharma G, Kumar A, Sharma S, Al-Saeedi SI, Al-Senani GM, Nafady A, Ahamad T, Naushad Mu, Stadler FJ. Fabrication of oxidized graphite supported La2O3/ZrO2 nanocomposite for the photoremediation of toxic fast green dye. J Mol Liq. 2019;277:738–48. https://doi.org/10.1016/j.molliq.2018.12.126.
Alzahrani E. Chitosan membrane embedded with ZnO/CuO nanocomposites for the photodegradation of fast green dye under artificial and solar irradiation. Anal Chem Insights. 2018;13:1–13. https://doi.org/10.1177/1177390118763361.
Acknowledgements
The authors thank Department of Chemistry, School of Sciences, Gujarat University for supporting this work.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
RKC: Conceptualisation; methodology, data curation, formal analysis, writing—original draft; PAS: Investigation; resources and supervision; PSS: Conceptualisation, critical reviewing and editing writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
All authors read and approved the final manuscript and gave consent for publication.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaudhari, R.K., Shah, P.A. & Shrivastav, P.S. Green synthesis of silver nanoparticles using Adhatoda vasica leaf extract and its application in photocatalytic degradation of dyes. Discover Nano 18, 135 (2023). https://doi.org/10.1186/s11671-023-03914-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-023-03914-5