Abstract
Background
We investigated the activity of loureirin B against liver fibrosis and the underlying molecular mechanisms.
Methods
Hepatic stellate cells (HSCs) from Sprague-Dawley rats were treated with different concentrations of loureirin B. We used the MTT assay to determine HSC proliferation, flow cytometry to analyze apoptosis, and western blot to determine the expressions of Bax, Bcl-2, Wnt1 and β-catenin. Real-time PCR was used to determine the expressions of Wnt1 and miR-148-3p.
Results
The MTT assay showed that loureirin B treatment significantly inhibited the proliferation of HSCs in time- and dose-dependent manners. Loureirin B significantly promoted the apoptosis of HSCs, increased the expression of Bax and decreased the Bcl-2 level. Western blot analysis showed that the expressions of Wnt1 and β-catenin were obviously lower in the loureirin B treatment group than in the control group. We also found that loureirin B could decrease the Wnt1 mRNA level and increase miR-148-3p expression. Knockdown of miR-148-3p using inhibitor could reverse the effects of loureirin B on the proliferation and apoptosis of HSCs and the expressions of Bax, Bcl-2, Wnt1 and β-catenin.
Conclusion
Our results suggest that loureirin B inhibited the proliferation and promoted the apoptosis of HSCs, and suppressed the Wnt/β-catenin signaling pathway via regulation of miR-148-3p.
Similar content being viewed by others
Background
Liver fibrosis is caused by repeated healing, repair and interstitial reconstruction after chronic liver injury. The activation of hepatic stellate cells (HSCs) is a critical event in liver fibrosis because these cells are the primary source of extracellular matrix in the injured liver [1]. Promoting HSC apoptosis and inhibiting HSC activation, proliferation and extracellular matrix production are important therapeutic approaches in cases of liver fibrosis. As the Wnt/β-catenin signaling pathway plays a vital role in the proliferation, differentiation, division, migration and apoptosis of HSCs, aberrant activation of this pathway may induce hepatic fibrogenesis. It was recently reported that blocking the Wnt/β-catenin signaling pathway is a crucial method to treat hepatic fibrogenesis [2, 3].
Wnt1 is a key member of the canonical Wnt/β-catenin signaling pathway, which activates the disheveled proteins, inhibits GSK-3 kinase, and accumulates β-catenin in the nucleus, and finally activates the β-catenin target genes. In this study, we explored the roles and molecular mechanisms for loureirin B in liver fibrosis. Our results indicate that loureirin B inhibited the proliferation and promoted the apoptosis of activated HSCs, and inhibited the Wnt signaling pathway via regulation of miR-148-3p.
Methods
Preparation, culture, identification and treatment of HSCs
Sixteen 400- to 450-g male Sprague-Dawley rats (specific pathogen free, from the Laboratory Animal Center of Chinese Academy of Sciences) were used in this study. Isolation of HSCs was performed as previously described [4]. Briefly, the liver was digested and perfused with collagenase IV (Worthington Biochemical Corporation), chain protease E and DNaseI solution, and then HSCs were isolated from the non-parenchymal liver cell (NPLC) fraction using 12% Nycodenz density gradient centrifugation (Nycodenz).
The primary HSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) High Glucose (Gibco) supplemented with 20% fetal bovine serum (FBS; GE Healthcare Life Sciences), 100 μg/ml penicillin and 100 μg/ml streptomycin, at 37 °C in a humid incubator containing 5% CO2. HSCs were identified via vitamin A lipid droplet auto-fluorescence using a fluorescence microscopy (Nikon Corporation), and HSC viability and purity were found to be above 90%. Activated HSCs were identified with immunofluorescence staining of α-SMA, which was the marker of activated HSCs after 1 week [5], and then treated with loureirin B. This study was approved by the Medical Ethics Committee of the First People’s Hospital of Yunnan Province.
Loureirin B and inhibitor of miR-148-3p
Loureirin B was obtained from the National Institute for the Control of Pharmaceutical and Biological Products of China, and reconstituted in DMSO at a final stock concentration of 20 mg/ml. The inhibitor of miR-148-3p was produced by GenePharma.
Detection of cell proliferation
Activated HSCs were cultured at a density of 105 per ml in DMEM High Glucose supplemented with 10% FBS in 96-well plates (100 μl/well), and then treated with 12.5, 25, 50, 100, 200 or 400 ng/μl loureirin B (concentrations based on the results of previous studies) [6, 7]. Two hours before the end of the incubation, 20 μl of the above-mentioned MTT solution was added to each well. Incubation was at 37 °C for 2 h. The inhibitory rates of activated HSC (aHSC) proliferation were determined by measuring absorbance at 570 nm.
Detection of apoptosis
The Apoptosis Detection Kit (BD Biosciences) was used to detect the apoptosis of HSCs. Activated HSCs that were treated with loureirin B were washed twice with cold PBS, and then suspended using 1× binding buffer at a concentration of 1 × 106 cells/ml. Then 100 μl of the solution (1 × 105 cells) was added to 5 ml culture, followed by 5 μl PI and 5 μl of FITC Annexin V. This was incubated for 15 min at room temperature in the dark, then 400 μl of 1× binding buffer was added to each tube. Analysis was done with a FACScan Cytometer (BD Accuri C6; BD Biosciences).
Western blot analysis
Specific antibodies of Wnt1, β-catenin, Bax and Bcl-2 (Santa Cruz Biotechnology) were used for western blot. HSCs were washed three times with ice-cold PBS and lysed in 100 to 500 μl RIPA lysis buffer (p0013B, Beyotime, supplemented with 1% PMSF). Cell debris was removed by centrifugation at 13,000×g for 30 min at 4 °C. Protein samples were boiled with 5× SDS-PAGE loading buffer for 10 min and subjected to electrophoresis in denaturing 10% SDS-polyacrylamide gel. Then proteins were transferred onto a polyvinylidene fluoride membrane (Millipore). The membrane was washed with Tris-buffered saline Tween (TBST), and blocked with 5% bovine serum albumin (BSA). The image was quantitatively analyzed using FluorChem V2.0 software.
Real-time PCR
Real-time PCR was performed to detect the relative expression level of Wnt1. PCR was performed in a total volume of 20 μl, including 10 μl of 2× Power SYBR Green PCR Master Mix (Applied Biosystems), 2 μl of cDNA (5 ng/μl) and 1 μl of primer mix (10 μM each). PCR amplification and detection were performed in a LightCycler 480 II (Roche Applied Science) as follows: an initial denaturation at 95 °C for 10 min; and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The relative gene expression was calculated using the comparative CT Method. The gene expressions of the target gene were normalized to an endogenous reference (GAPDH), and their values relative to the calibrator were obtained with the formula 2−ΔΔCt. ΔCT was calculated by subtracting the average GAPDH CT from the average CT of the gene of interest. The ratio defines the level of relative expression of the target gene to that of GAPDH.
Hairpin-it miR-148-3p qRT-PCR Primer Set (GenePharma) was used for the measurement of the relative quantity of miR-148-3p. The mRNA expression of miR-148-3p was normalized to the endogenous expression of U6.
Statistical analysis
Statistical analyses were performed using SSPS18.0 software. Data are presented as means ± standard deviations (ranges). The differences between the control group and treatment group were compared with the Student’s t-test. p < 0.05 was regarded as statistically significant.
Results
Separation and identification of HSCs
We used an inverted phase contrast microscope to observe the primary quiescent HSCs separated from the rat liver. It is easy to see the spontaneous green fluorescence, but it disappeared quickly. The cells also contained many refractive granules. After culture for about 1 week, the HSCs changed their phenotype from quiescent to activated. Immunofluorescence staining showed that the positive expression of α-smooth muscle actin (α-SMA) was above 90%, and the shapes of aHSC changed to a star (not shown).
Loureirin B inhibits the proliferation and promotes the apoptosis of HSCs and downregulates the expression of Bcl-2
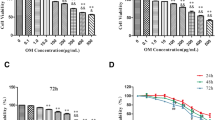
We treated the HSCs with loureirin B, and found that it significantly inhibited their proliferation in a dose-dependent manner (Fig. 1a). We also assessed the inhibitory effects of loureirin B in different time, and the results showed that loureirin B also inhibited the proliferation of HSCs in a time dependent manner (Fig. 1b). Using flow cytometry assay, we further found that loureirin B could promote the apoptosis of HSCs in a dose dependent manner (Fig. 2a and b). Western blotting assay showed that loureirin B increased the expression of Bax and decreased the level of Bcl-2, and the expression of Bcl-2 in 100 ng/μl loureirin B group was lower than that in 50 ng/μl loureirin B group (Fig. 3a).
Loureirin B inhibits the proliferation of HSCs. a Activated hepatic stellate cells (aHSCs) were treated with 12.5, 25, 50, 100, 200 or 400 ng/μl loureirin B for 48 h, and then detected using the MTT assay. b aHSCs were treated with 100 ng/μl loureirin B for 24, 48 and 72 h, and then detected using the MTT assay. The experiments were repeated three times. *p < 0.05; **p < 0.01; ***p < 0.001
Loureirin B promotes the apoptosis of HSCs. a Activated hepatic stellate cells (aHSCs) were treated with 50 and 100 ng/μl loureirin B for 48 h, and then assessed using flow cytometry. b Statistical analysis of flow cytometry results. Data are the means ± SEM from four independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
Loureirin B increased Bax expression, decreased Bcl-2 expression, and inactivated the Wnt/β-catenin signaling pathway. a and b Effect of loureirin B on the expression levels of Bax, Bcl-2, Wnt1 and β-catenin, as detected via western blot assay and statistically analyzed using Student’s t-test. Data are the means ± SEM from four independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
Loureirin B inhibits the proliferation and promotes the apoptosis of HSCs, and inhibits the Wnt/β-catenin signaling pathway via regulation by regulating miR-148-3p
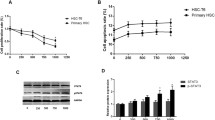
Activating the Wnt/β-catenin signaling pathway was reported to promote liver fibrosis [8], so we explored whether loureirin B affected this pathway. Our western blot results showed that loureirin B significantly reduced the protein expression levels of Wnt1 and β-catenin (Fig. 3b), and that 100 ng/μl loureirin B had a higher inhibitory effect on the expressions of Wnt1 and β-catenin than 50 ng/μl loureirin B (Fig. 3b). Using real-time PCR, we found that loureirin B also decreased the mRNA level of Wnt1 (Fig. 4a). Using TargetScan, we predicted that miR-148-3p could target Wnt1.
Loureirin B decreased the expression of Wnt1 via upregulation of miR-148-3p. a and b The expression levels of Wnt1 and miR-148-3p were detected using real-time PCR. c Using the miR-148-3p inhibitor to silence miR-148-3p. d Knockdown of miR-148-3p increased the loureirin B-decreased Wnt1 mRNA level detected using real-time PCR. Data are the means ± SEM from four independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
Then we detected the expression of miR-148-3p in the HSCs treated with loureirin B. The results showed that loureirin B significantly increased miR-148-3p expression (Fig. 4b). To confirm that loureirin B inhibited the Wnt/β-catenin signaling pathway via regulation of miR-148-3p, we used an inhibitor to reduce its expression in the loureirin B-treated HSCs (Fig. 4c). The results showed that knockdown of miR-148-3p increased the Wnt1 mRNA and protein levels that had been reduced by loureirin B treatment (Figs 4d and 5a). We also found that silencing the miR-148-3p in loureirin B-treated cells could reverse the effects of loureirin B on the expressions of β-catenin, Bax and Bcl-2 (Fig. 5a and b).
Loureirin B regulated the Wnt/β-catenin signaling pathway and the expressions of Bax and Bcl-2 via upregulation of miR-148-3p. a Knockdown of miR-148-3p increased the loureirin B-decreased Wnt1 and β-catenin, as detected via western blot. b Knockdown of miR-148-3p downregulated the loureirin B-increased Bax and upregulated the loureirin B-decreased Bcl-2 expression. Data are the means ± SEM from four independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
These results indicate that loureirin B inhibited the Wnt/β-catenin signaling pathway via regulation of miR-148-3p. Silencing miR-148-3p could reduce loureirin B-induced apoptosis and enhance loureirin B-suppressed proliferation of HSCs (Fig. 6a and b). These results indicate that loureirin B affected the proliferation and apoptosis of HSCs via regulation of miR-148-3p.
Loureirin B inhibits the proliferation and promotes the apoptosis of HSCs via regulation of miR-148-3p. a and b Flow cytometry was used to analyze the apoptosis of HSCs when inhibitor was applied to silence the miR-148-3p expression. c The proliferation of HSCs was detected using the MTT assay. Data are the means ± SEM from four independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
The activation of HSCs is considered an important event in liver fibrosis, and epithelial–mesenchymal transition (EMT) plays an important role in HSC activation [9]. HSCs have several important functions under both physiological and pathological conditions associated with sinusoids [10]. In the healthy liver, HSCs have two morphologically distinguishable forms: the quiescent HSCs characterized by small cell size and low proliferating activity [11]; and the activated form [12, 13]. Under physiological conditions, quiescent HSCs store about 75% of total body vitamin A in lipid droplets in the cytoplasm, participate in the metabolism of extracellular matrix in liver sinusoids, and regulate the bleeding functions of sinusoids [10, 14, 15]. In response to liver injury, HSCs change from quiescent to activated [12, 13], with this transition is caused by the inflammatory factors, such as TGF-β1, PDGF, VEGF, TNF and so on, which were produced by immune cells, Kupffer cells and endothelial cells. Activated HSCs proliferate vigorously, lose vitamin A, express α-SMA, and synthesize a large amount of extra-cellular matrix (ECM) components [16]. The activated HSCs also change to fibroblasts or myofibroblasts [17]. It is clear that TGF-β1 could promote HSC activation [18]. HSCs could produce and secrete TGF-β1 through autocrine and paracrine manners, while TGF-β1 could promote HSC proliferation and accumulation of ECM in the liver, leading to liver fibrosis [19].
Resina draconis is a special resin derived from the fruit and stem of certain palm trees, such as Dracaena cochinchinensis (Lour.) S. C. Chen, with the efficacy of promoting blood circulation and angiogenesis, diminishing inflammation, relieving pain and stopping bleeding. Loureirin B is one of the most important chemical compositions and physiologically active ingredients of resina draconis. It has the molecular structure propan-1-one, 1-(4-hydroxyphenyl)-3-(2, 4, 6-trimethoxyphenyl)- 1-(4-hydroxyphenyl)-3-(2, 4, 6-trimethoxyphenyl) propan-1-one.
Jiang et al. reported that loureirin B was the inhibitor of PAI-1 (IC50 = 26.10 μM) which was the natural inhibitor of tissue-type and urokinase-type plasminogen activators. Loureirin B could inhibit the formation of the PAI-1/uPA complex [20]. Loureirin B could promote insulin secretion by upregulating the mRNA expressions of Pdx-1, MafA and the intracellular ATP level, and inhibiting the KATP current [17]. He et al. reported that loureirin B downregulated p-ERK and p-JNK in TGF-β1-stimulated fibroblasts and cultured hypertrophic scar tissue ex vivo, and further affected the expressions of Col1 and FN [6]. In hypertrophic scar fibroblasts, loureirin B could dose-dependently reduce the mRNA and protein levels of type I collagen (CoII), type III collagen (ColIII) and α-smooth muscle actin (α-SMA) by regulating MMPs and TIMPs, inhibit scar fibroblast proliferation and suppress TGF-β1-induced fibrosis, possibly via TGF-β1/Smad2/3 pathway [7]. Our study found that loureirin B inhibited HSC proliferation, promoted Hsc apoptosis, and suppressed the Wnt signaling pathway via regulating miR-148-3p.
The Wnt/β-catenin signaling pathway plays important roles in HSC activation [21]. Wnt1 is an essential component of the canonical Wnt/β-catenin signaling pathway, which leads to the activation of disheveled proteins, inhibition of GSK-3β kinase, nuclear accumulation of β-catenin, and finally activation of Wnt target genes [22]. Inhibiting the expression of Wnt1 maybe an attractive method to block the Wnt/β-catenin signaling pathway, thereby inhibiting the activation of HSCs.
Conclusions
Our findings indicate that loureirin B inhibited HSC proliferation, promoted HSC apoptosis, and downregulated the expression of Bcl-2. Very interestingly, loureirin B inhibited the Wnt/β-catenin signaling pathway via regulation of miR-148-3p in HSCs.
There have been no reports about the correlation between miR-148-3p and HSC function. Our results found that miR-148-3p was involved in the regulation of lourerin B on HSC proliferation and apoptosis. Our findings suggest that three major aspects of loureirin B activity – inhibition of HSC proliferation, promotion of HSC apoptosis, and inactivation of the Wnt/β-catenin signaling pathway – occur via regulation of miR-148-3p.
Abbreviations
- ECM:
-
Extra-cellular matrix
- HSCs:
-
Hepatic stellate cells
- NPLCs:
-
Non-parenchymal liver cells
- α-SMA:
-
α-smooth muscle actin
References
Lan T, Li C, Yang G, Sun Y, Zhuang L, Ou Y, Li H, Wang G, Kisseleva T, Brenner D, Guo J. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology. 2018; https://doi.org/10.1002/hep.29885.
Yang MD, Chiang YM, Higashiyama R, Asahina K, Mann DA, Mann J, Wang CC, Tsukamoto H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology. 2012;55:1271–81. https://doi.org/10.1002/hep.24792.
Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56. https://doi.org/10.1146/annurev-pathol-011110-130246.
Shi MN, Huang YH, Zheng WD, Zhang LJ, Chen ZX, Wang XZ. Relationship between transforming growth factor beta1 and anti-fibrotic effect of interleukin-10. World J Gastroenterol. 2006;12:2357–62.
Qin S, Jiang H, Su S, Wang D, Liang Z, Zhang J, Yang W. Inhibition of hepatic stellate cell proliferation by bone marrow mesenchymal stem cells via regulation of the cell cycle in rat. Exp Ther Med. 2012;4:375–80. https://doi.org/10.3892/etm.2012.628.
He T, Bai X, Yang L, Fan L, Li Y, Su L, Gao J, Han S, Hu D. Loureirin B Inhibits Hypertrophic Scar Formation via Inhibition of the TGF-beta1-ERK/JNK Pathway. Cell Physiol Biochem. 2015;37:666–76. https://doi.org/10.1159/000430385.
Bai X, He T, Liu J, Wang Y, Fan L, Tao K, Shi J, Tang C, Su L, Hu D. Loureirin B inhibits fibroblast proliferation and extracellular matrix deposition in hypertrophic scar via TGF-beta/Smad pathway. Exp Dermatol. 2015;24:355–60. https://doi.org/10.1111/exd.12665.
Xiang T, Zhang S, Cheng N, Ge S, Wen J, Xiao J, Wu X. Oxidored-nitro domain-containing protein 1 promotes liver fibrosis by activating the Wnt/beta-catenin signaling pathway in vitro. Mol Med Rep. 2017;16:5050–4. https://doi.org/10.3892/mmr.2017.7165.
Zheng J, Wang W, Yu F, Dong P, Chen B, Zhou MT. MicroRNA-30a Suppresses the Activation of Hepatic Stellate Cells by Inhibiting Epithelial-to-Mesenchymal Transition. Cell Physiol Biochem. 2018;46:82–92. https://doi.org/10.1159/000488411.
Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–35. https://doi.org/10.1055/s-2001-17550.
Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–35. https://doi.org/10.1056/NEJM199306243282508.
Casu A, Canepa M, Nanni G. Perisinusoidal stellate cells or Ito cells and their role in hepatic fibrosis. Pathologica. 1994;86:467–99.
Senoo H, Sato M, Imai K. Hepatic stellate cells--from the viewpoint of retinoid handling and function of the extracellular matrix. Kaibogaku Zasshi. 1997;72:79–94.
Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985;160:138–49.
Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–53.
Senoo H, Imai K, Matano Y, Sato M. Molecular mechanisms in the reversible regulation of morphology, proliferation and collagen metabolism in hepatic stellate cells by the three-dimensional structure of the extracellular matrix. J Gastroenterol Hepatol. 1998;13:S19–32. https://doi.org/10.1111/jgh.1998.13.s1.19.
Sha Y, Zhang Y, Cao J, Qian K, Niu B, Chen Q. Loureirin B promotes insulin secretion through inhibition of KATP channel and influx of intracellular calcium. J Cell Biochem. 2018;119:2012–21. https://doi.org/10.1002/jcb.26362.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. https://doi.org/10.1002/path.2277.
Mabuchi A, Mullaney I, Sheard PW, Hessian PA, Mallard BL, Tawadrous MN, Zimmermann A, Senoo H, Wheatley AM. Role of hepatic stellate cell/hepatocyte interaction and activation of hepatic stellate cells in the early phase of liver regeneration in the rat. J Hepatol. 2004;40:910–6. https://doi.org/10.1016/j.jhep.2004.02.005.
Jiang Y, Zhang G, Yan D, Yang H, Ye Z, Ma T. Bioactivity-Guided Fractionation of the Traditional Chinese Medicine Resina Draconis Reveals Loureirin B as a PAI-1 Inhibitor. Evid Based Complement Alternat Med. 2017;2017:9425963. https://doi.org/10.1155/2017/9425963.
Sun H, Chen G, Wen B, Sun J, An H, Pang J, Xu W, Yang X, He S. Oligo-peptide I-C-F-6 inhibits hepatic stellate cell activation and ameliorates CCl4-induced liver fibrosis by suppressing NF-kappaB signaling and Wnt/beta-catenin signaling. J Pharmacol Sci. 2018; https://doi.org/10.1016/j.jphs.2018.01.003.
Daniels DL, Eklof Spink K, Weis WI. beta-catenin: molecular plasticity and drug design. Trends Biochem Sci. 2001;26:672–8.
Funding
National Natural Science Foundation of China (No. 81560107 and 81160062) and the Science and Technology Application Project of Yunnan Province (No. 2014FB091).
Availability of data and materials
All the data were generated during the study.
Author information
Authors and Affiliations
Contributions
SZJ designed the research. HJP, TM, LYL, XLT, AY, LT, ZR and SZJ performed the research. HJP, SZZ and SZJ analyzed the data. HJP and SZJ wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the First People’s Hospital of Yunnan Province.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hu, JP., Zhang, R., Tang, M. et al. Loureirin B inhibits the proliferation of hepatic stellate cells and the Wnt/β-catenin signaling pathway by regulating miR-148-3p. Cell Mol Biol Lett 23, 35 (2018). https://doi.org/10.1186/s11658-018-0098-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11658-018-0098-9