Abstract
Background
Management of uncontained medial proximal tibial defects during primary total knee arthroplasty (TKA) can be challenging, especially for defects ≥ 10 mm in depth. This study sought to assess the outcomes of autogenous structural bone grafts to address these defects.
Materials and methods
In this prospective study, patients with uncontained medial proximal tibial defects ≥ 10 mm in depth undergoing TKA were managed by autogenous structural bone grafts fixed by screws and were followed up for at least 36 months. Patients were followed-up clinically with Knee Society Score (KSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Additionally, radiological follow-up was done to assess bone graft union and implant stability.
Results
The study included 48 patients with a mean age of 69.2 ± 4.5 years. The mean body mass index (BMI) was 31.4 ± 3.7 kg/m2. The mean defect depth was 17 ± 3.6 mm. With a mean follow-up period of 52.2 ± 12.3 months, the median KSS improved significantly from 30 preoperatively to 89, P < 0.001. The median WOMAC score reduced significantly from 85 preoperatively to 30.5, P < 0.001. The mean ROM increased significantly from 73 ± 12.4 preoperatively to 124 ± 8.4 degrees, P < 0.001. The mean graft union time was 4.9 ± 1 months. No significant complications were reported.
Conclusions
Autogenous bone graft reconstruction is a safe and effective method of addressing uncontained medial proximal tibial defects in primary TKA.
Level of evidence
Level IV.
Similar content being viewed by others
Introduction
Knee osteoarthritis (OA) is a common age-related disorder that may lead to significant pain, stiffness, and reduced knee function, especially in the advanced degrees [1, 2].
Total knee arthroplasty (TKA) is a widely employed intervention for treating end-stage knee OA, aiming to establish a knee that mimics the natural function and kinematics of the native knee [3, 4]. Adequate preoperative planning, achieving precise implant positioning, correcting the limb alignment, restoring the joint line, and ensuring gap balancing are imperative for the success of the TKA procedure [5, 6].
Patients experiencing advanced knee OA may present with a significant varus deformity, often accompanied by bone defects, especially in the posteromedial aspect of the tibia due to degenerative erosions. These uncontained defects do not provide peripheral support for the implant components [7, 8].
Addressing these bone defects is of paramount significance for the success of TKA [9, 10]. If not appropriately addressed, these defects can compromise the bone–implant interface, leading to implant components loosening and malalignment and a greater likelihood of requiring revision surgery [11]. After resection of the tibial plateau in TKA surgery, any defect exceeding 10 mm in its largest diameter typically requires reconstruction [9].
Bone defects can be managed in several ways depending on the defect size and its containment status after the tibial bone cut [7]. Bone cement is used to fill defects less than 5 mm deep. Additionally, the approach of increased tibial resection with the use of a thicker polyethylene insert may be applied for defects of less than 10 mm [12].
If bone deficiencies exceed 10 mm, it is advisable not to cut the tibia to the level of the deficit, as distal tibial resection weakens osseous support, leading to a decreased area of support and increased loading [13, 14]. Therefore, reconstruction using allograft, autograft, metal augments, cones, and metaphyseal sleeves should be employed [9, 10, 15, 16].
The use of metal augments to address uncontained defects ≥ 10 mm deep has been described with good functional outcomes [16]. However, this technique raises medical expenses, necessitates additional bone cutting involving the cortical bone, and can complicate future revision surgeries [15, 16].
Few knee arthroplasty studies have evaluated the use of autogenous or allogenous bone grafts in dealing with uncontained defects exceeding 10 mm in depth in the medial proximal tibia in primary cases [9, 13].
Therefore, this study was conducted to assess the functional and radiological results of structural autograft bone reconstruction of medial proximal tibial defects ≥ 10 mm deep in primary TKA and to investigate the preoperative factors affecting the results.
Material and methods
This study was a prospective study of patients with uncontained medial proximal tibial defects undergoing primary TKA between March 2015 and March 2020. Written consent from participants was obtained, along with approval from the institutional review board (IRB). Surgeries were performed by a single surgeon (A.A.D.).
Inclusion criteria were patients with Kellgren and Lawrence grade 4 (KL4) OA with varus deformity and uncontained medial proximal tibial defects ≥ 10 mm deep after the proximal tibial cut. Patients should have completed at least 36 months of follow-up to be included.
Patients with previous knee surgery, valgus OA, rheumatoid arthritis, infection, osteonecrosis, defects after tumor resection, contained defects, associated significant distal femur defects, Charcot knee, defects less than 10 mm depth, or use of metal augmentations were excluded.
Preoperative assessment
History taking was done, including analysis of symptoms of pain, stiffness, instability, up-stairing, down-stairing, gait, and rising from chair. The body mass index (BMI) was calculated, and patients were classified based on the World Health Organization (WHO) classification [17] as underweight (BMI < 18.5 kg/m2), healthy (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obesity class I (30–34.9 kg/m2), class II (35–39.9 kg/m2) or class III (≥ 40 kg/m2).
Complete limb examination was done with assessment of the varus deformity regarding the degree, whether correctable or fixed, and the associated deformities (flexion or rotatory). Additionally, the mediolateral instability and the lateral ligament laxity were assessed. Active and passive ranges of motion and patellar tracking were also evaluated.
Knee anteroposterior (AP) and lateral standing plain X-rays were done to confirm the clinical diagnosis of advanced arthritis and assess the site, size, containment, shape, and slope of the bone defects. The depth of bone defect from the expected level of the proximal tibial cut was preliminary assessed in the preoperative AP X-ray, Fig. 1.
Knee plain X-ray AP view showing measurement of the medial tibial defect. Line A is through the anatomical axis of the tibia. Line B passes through the deepest point of the defect and is perpendicular to line A. Line C passes through the highest point of the head fibula and is perpendicular to line A, representing the expected resection line of the tibial plateau. The red line is the size of the defect
Long-film AP weight-bearing X-ray was done to evaluate the anatomical and mechanical axes and quantify the degree of varus deformity by measuring the anatomical femorotibial angle (aFTA).
Additional X-rays included a skyline view at 30 degrees flexion for evaluation of the patellar maltracking and stress views for evaluation of the coronal instability due to bone stock loss or ligamentous insufficiency.
Surgical technique
Surgeries were done under combined epidural and spinal anesthesia. A standard medial parapatellar approach was used with traditional steps for preparation of the tibia and femur.
The proximal tibia was displaced anteriorly, and the tibial cut was done through the nondeficient lateral tibial plateau which was our reference in tibial cut by using a special stylus adjusted at 10 mm, with either intramedullary or extramedullary alignment guides. The tibial cut was done using an oscillating saw taking 10 mm from nonworn lateral tibial plateau leaving a defect in medial tibial plateau.
Dealing with the deficient medial tibial plateau was done using bone graft blocks from the proximal tibial or distal femoral bone cuts. Firstly, the concave and irregular surface of the defect was flattened by minimal bone removal using the oscillating saw with exposure of healthy bone to enhance further healing with the graft. The depth of the defect was evaluated and measured using a sterilized ruler.
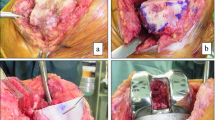
The graft was fashioned using bony rongeur, placed over the flattened defect, and secured provisionally by Kirschner wires (K-wires). The K-wires were then replaced by 3.5 mm cancellous screws, making sure that they did not interfere with the tibial component keel or stem. The protruding part of the graft was then removed by an oscillating saw to create a flat upper tibial surface, Fig. 2.
Intraoperative images of the bone graft preparation and insertion into the defect. A Evaluation of the defect after the proximal tibial cut. B Placement of the graft into the defect and provisional fixation by two K-wires. C Replacement of the two K-wires with two 3.5 mm partially threaded cancellous screws. D Removing the protruding part of the graft with an oscillating saw to create a flat upper tibial surface
Before cementation, the interface of the bone graft and the tibia was filled by impaction bone graft to avoid the extrusion of cement into this interface.
Trial components were inserted for assessment of size, prosthesis fitting, position, equality of bone gaps, and traditional restoration of neutral mechanical alignment was important as it had great effect on bone graft survival and prosthesis loosening. Tibial stem was used in all cases to protect the bone graft from stress.
The definitive prosthesis was inserted by the routine cementing technique. Posterior-stabilized (PS) TKA with a stemmed tibial component was used to unload the deficient metaphyseal bone. In cases with severe lateral collateral ligament laxity, Legacy Constrained Condylar Knee (LCCK) prosthesis (Zimmer-Biomet, Warsaw, Indiana, USA) was used.
Patelloplasty was done by removing all the osteophytes by the nibbler and denervation of the patella by applying cautery circumferentially around the patella (patellar circumcision).
Good hemostasis was achieved after the release of the tourniquet, followed by closure of the wound after application of a suction drain.
Postoperative care and follow-up
Epidural postoperative analgesia was given in the ward using a continuous syringe pump system for sustained analgesia for 48 h postoperatively. Additionally, intravenous antibiotics were given for 48 h postoperatively. Static quadriceps and hamstring muscle strengthening exercises and straight leg raising exercises were commenced from day one, in addition to active and assisted flexion–extension range of motion (ROM) exercises. Weight-bearing was permitted without limitations.
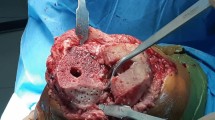
Patients were followed up at 6 weeks, 3 months, and 6 months, then yearly, and were evaluated clinically with Knee Society Score (KSS) [18] and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [19]. Follow-up knee X-rays were also done at 6 weeks, 3 months, 6 months, and then yearly to assess component stability and bone graft union, Fig. 3.
A 58-year-old male with advanced left knee OA. A Preoperative X-rays showing advanced OA and the medial proximal tibial defect. B Preoperative standing photographs showing varus deformity. C Postoperative X-rays after TKA using LCCK and reconstruction of the defect using structural autograft block fixed with two screws. D Three-year follow-up X-rays showing complete union of the graft
Statistical analysis
Data were analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp).
Qualitative data were expressed as numbers and percentages. Quantitative data were expressed as mean, and standard deviation (SD) when normally distributed and as median and interquartile range (IQR) when not normally distributed.
Comparison of preoperative and postoperative continuous data was done using the paired samples t-test or the Wilcoxon signed-rank test when appropriate. Comparison of the effect of different variables on the functional and radiological outcomes was done using the Student’s t-test, the Mann–Whitney U-test, the one-way ANOVA, or the Kruskal–Wallis H-test, when applicable. Significance of the obtained results was judged at the 5% level.
Results
Demographics and baseline characteristics
This study included 48 patients, including 25 (52.1%) patients < 70 years and 23 (47.9%) patients ≥ 70 years, with a mean age of 69.2 ± 4.5 (range, 58–78) years. Among the participants, 18 (37.5%) were males and 30 (62.5%) were females. The mean BMI was 31.4 ± 3.7 (range, 25.4–41.5) kg/m2. Regarding the BMI classification, 21 (43.8%) patients had obesity class I, and 19 (39.6%) patients were overweight.
TKA was done on the left knee in 27 (56.3%) patients and the right knee in 21 (43.8%) patients.
Regarding the preoperative aFTA, 31 (64.6%) patients had a 10–25° varus angulation, while 17 (35.4%) patients had > 25° varus angulation. Additionally, 22 (45.8%) patients had a flexion contracture of > 10°, Table 1.
The mean depth of defect as measured intraoperatively was 17 ± 3.6 (range, 10–20) mm. The mean thickness of the used polyethylene insert was 13 ± 1.7 (range, 10–16) mm. The average operative time was 129.3 ± 6.7 (range, 120–140) minutes. Primary TKA prosthesis with stemmed tibial component was used in 38 (79.2%) patients, and LCCK prosthesis was used in 10 (20.8%) patients with severe lateral ligament laxity.
Functional and radiological outcomes
The average follow-up period was 52.2 ± 12.3 (range, 36–96) months. The median KSS improved significantly from 30 (IQR, 27–35) preoperatively to 89 (IQR, 85–93) at the last follow-up, P < 0.001. Additionally, there was a significant reduction in the median WOMAC score from 85 (IQR, 80–90) preoperatively to 30.5 (IQR, 27–35) at last follow-up, P < 0.001.
The mean flexion–extension ROM increased significantly from 73 ± 12.4 (range, 45–100) degrees preoperatively to 124 ± 8.4 (range, 95–135) degrees at the last follow-up, P < 0.001. In addition, there was significant correction of the varus malalignment and the flexion contractures, P < 0.001, Table 2. The mean graft union time was 4.9 ± 1 (range, 3–8) months.
Factors affecting the outcomes
At the last follow-up, patients younger than 70 years had a significantly higher median KSS score than those ≥ 70 years, 90 (IQR, 85–93) and 85 (IQR, 83–90), respectively, P = 0.016. Additionally, the score was higher in overweight patients, 90 (IQR, 87–93), compared with patients with obesity class I, 88 (IQR, 85–90) and obesity class II, 85 (IQR, 85–89), P = 0.049, Table 3.
Regarding the last follow-up WOMAC, patients with obesity class II had a significantly higher median WOMAC, 40 (IQR, 37.5–40.2), compared with overweight patients, 27.3 (IQR, 27–33, and patients with obesity class I, 31 (IQR, 27–35), P = 0.007, Table 4.
At the last follow-up, males had a significantly higher mean flexion–extension ROM compared with females, 128.3 ± 5.9 (range, 110–135) degrees and 121.3 ± 8.7 (range, 95–130), P = 0.004. Overweight patients had a significantly higher mean ROM, 127.4 ± 4 (range, 120–135), than patients with obesity class I, 124 ± 7.8 (range, 110–135) and obesity class II, 118.6 ± 8.5 (range, 105–130) degrees, P = 0.020, Table 5.
The mean graft union time was higher in patients ≥ 70 years, 5.3 ± 1.0 (range, 4–8), compared with patients < 70 years, 4.5 ± 0.8 (range, 3–6) months, P = 0.002, Table 6.
Complications
Persistent medial side joint pain occurred in two (4.2%) patients, most probably due to pes anserine bursitis. Additionally, two (4.2%) patients had delayed graft union at 7 and 8 months.
Discussion
Patients with advanced knee OA and varus deformity usually have uncontained proximal tibial bone defects, which are a technically demanding aspect of primary TKA [20]. Achieving successful outcomes relies on properly positioning and aligning the implant components [21].
In this study, uncontained medial tibial bone defects were managed by autograft reconstruction, taken from the distal femur cuts. With a mean follow-up of 52.2 months, there was a significant improvement in the KSS and WOMAC scores and the flexion–extension ROM, with significant correction of the varus malalignment and the flexion contracture. Better functional scores were observed in patients < 70 years and patients with lower BMI. A better range of motion was observed in male patients and patients with lower BMI. Graft union was faster in patients < 70 years.
Autogenous structural bone grafts have been described as a treatment option for uncontained tibial bone defects of 5–10 mm in diameter, with good long-term outcomes [22].
In our study, significant improvement of WOMAC scores was achieved in the form of reduction from 85 preoperatively to 30.5, with an average follow-up of 52.2 months. Additionally, the median KSS improved significantly from 30 before surgery to 89 at last follow-up.
Chon et al. [13] used autogenous structural and cancellous chip bone graft for the reconstruction of medial proximal tibial defects of 10 mm or more in depth in 40 patients undergoing primary TKA. With At least 1 year of follow-up, a significant improvement in WOMAC scores was achieved [13]. Yoon et al. [10] reported the outcomes of using autogenous onlay bone graft in 19 patients (22 knees) with an average 12-mm-deep uncontained medial tibial bone defects undergoing primary TKA. With a mean follow-up period of 30.2 months, there was a significant increase in the mean KSS score from 30 preoperatively to 92 at the last follow-up [10].
In our study, significant improvement of knee ROM was achieved. Similarly, Chon et al. [13] reported significant improvement in the ROM.
In our study, bony union was achieved in all cases at final follow-up, with a mean graft union time of 4.9 ± 1 months. Similarly, Chon et al. [13] reported that all cases showed bone union at the graft-bone interface. Yoon et al. [10] reported a mean time of 3.2 months for solid union. Kharbanda and Sharma [23] reported an average graft incorporation time of 4.5 months in 54 knees in their study of autograft reconstructions for bone defects with an average follow-up of 7.8 years.
In the current study, no significant complications were reported, such as infection, implant loosening or graft nonunion. Revision surgery was not required in any patients. Similarly, no significant complications were reported in Chon et al. [13] and Yoon et al. [10] studies.
In the standard tibial component of TKA, 53–67% of load sharing occurs at the cortical rim [24]. Thus, uncontained defects without cortical support must be addressed adequately to ensure prosthesis stability [25].
In general, it is recommended to use bone grafting for defects of 5–10 mm, while metal augmentation has been described to address these uncontained defects, especially for defects exceeding 10 mm [26,27,28]. Nevertheless, achieving anatomical bone reconstruction is not feasible with metal augments, necessitating unavoidable extra bone resection to allow a proper fit [29]. Additionally, persistent knee pain may result from augment protrusion [12]. Metal blocks also result in substantial bone defects in subsequent revision surgeries [11].
The use of structural allografts has also been described [9]. However, several drawbacks exist, including graft nonunion, collapse or resorption, in addition to the risk of disease transmission such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), and human T-lymphotropic virus (HTLV) [30, 31]. Iwase et al. [9] reported a case of nonunion and another case with radiolucent line out of 17 patients treated with allogenous structural bone graft.
Autogenous bone grafting has the advantage of providing biological stability while reinforcing the bone stock and lowering medical costs [32].
Using structural autogenous bone grafting for uncontained bone defects ≥ 10 mm deep, we have obtained good outcomes without significant complications such as infection, graft nonunion, graft resorption, or implant loosening. Using local autograft blocks from the knee cuts avoids donor site morbidity in other anatomical areas of the skeleton.
A stemmed tibial component and stable graft fixation with screws are paramount to ensure the stability of the prosthesis. Watanabe et al. [32] reported a 100% autograft union rate in 30 patients without using screws. However, using cancellous screws for fixing autologous bone grafts allows robust initial fixation, therefore achieving a high rate of bony union and implant stability [33].
The current study has limitations, such as the lack of control group. Preoperative computed tomography (CT) scans were not done to measure volumetric loss. Follow-up CT scans were not done to assess the cross-trabeculation between the graft and the proximal tibial bone. Additionally, we measured only the depth of the defect, with the inability to measure the volume of the defect intraoperatively. The defects have variable sizes and shapes, and there should be a specific device that is able to measure the volume intraoperatively.
Future comparative studies between structural autogenous bone grafts and other options, such as structural allografts or metal augments should be conducted to further recommend the ideal method of reconstruction of uncontained tibial defects with a depth ≥ 10 mm. Also, a long-term follow-up study is needed to assess the potential complications associated with screw fixation and its impact on the stability and durability of the implant.
Conclusions
Uncontained medial proximal tibial defects ≥ 10 mm deep in patients undergoing primary TKA can be adequately managed using structural autogenous bone graft fixed with screws. This technique has satisfactory clinical and radiological outcomes without significant complications.
Availability of data and materials
The dataset analyzed in this study is available from the corresponding author on request.
References
Katz JN, Arant KR, Loeser RF (2021) Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325(6):568–578. https://doi.org/10.1001/jama.2020.22171
Kloppenburg M, Berenbaum F (2020) Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage 28(3):242–248. https://doi.org/10.1016/j.joca.2020.01.002
Budhiparama NC, Lumban-Gaol I, Novito K, Hidayat H, De Meo F, Cacciola G, Cavaliere P (2023) PCL retained is safe in medial pivot TKA-a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-023-07634-2
Li Z, Chen X, Wang X, Zhang B, Wang W, Fan Y, Yan J, Zhang X, Zhao Y, Lin Y, Liu J, Lin J (2022) HURWA robotic-assisted total knee arthroplasty improves component positioning and alignment - A prospective randomized and multicenter study. J Orthop Translat 33:31–40. https://doi.org/10.1016/j.jot.2021.12.004
Zhang Z, Luo Y, Zhang C, Wang X, Zhang T, Zhang G (2023) Prediction of gap balancing based on 2-D radiography in total knee arthroplasty for knee osteoarthritis patients. Arthroplasty 5(1):60. https://doi.org/10.1186/s42836-023-00218-y
Stobe C, Hoechel S, Muller-Gerbl M, Nowakowski AM (2020) Systematic effects of femoral component rotation and tibial slope on the medial and lateral tibiofemoral flexion gaps in total knee arthroplasty. J Orthop Translat 24:218–223. https://doi.org/10.1016/j.jot.2019.09.004
Aggarwal AK, Baburaj V (2021) Managing bone defects in primary total knee arthroplasty: options and current trends. Musculoskelet Surg 105(1):31–38. https://doi.org/10.1007/s12306-020-00683-7
Ho JPY, Cho JH, Nam HS, Park SY, Lee YS (2023) Constitutional alignment predicts medial ligament balancing in mechanically aligned total knee arthroplasty for varus knees. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-023-07660-0
Iwase D, Metoki Y, Kusumoto Y, Aikawa J, Fukushima K, Takano S, Mukai M, Uchida K, Inoue G, Takaso M (2022) Using allogenous structural bone graft for uncontained tibial bone defects >/= 10 mm in depth in primary total knee arthroplasty. BMC Musculoskelet Disord 23(1):528. https://doi.org/10.1186/s12891-022-05491-7
Yoon JR, Seo IW, Shin YS (2017) Use of autogenous onlay bone graft for uncontained tibial bone defects in primary total knee arthroplasty. BMC Musculoskelet Disord 18(1):502. https://doi.org/10.1186/s12891-017-1826-4
Lei PF, Hu RY, Hu YH (2019) Bone Defects in Revision Total Knee Arthroplasty and Management. Orthop Surg 11(1):15–24. https://doi.org/10.1111/os.12425
Cuckler JM (2004) Bone loss in total knee arthroplasty: graft augment and options. J Arthroplasty 19(4 Suppl 1):56–58. https://doi.org/10.1016/j.arth.2004.03.002
Chon JG, Kang JW, Kim CU, Jeong U, Go J (2021) Treatment of 10-mm-deep or greater uncontained tibial bone defects in primary total knee reconstruction without metal augmentation: autologous oblique structural peg bone and cancellous chip bone grafting. Clin Orthop Surg 13(2):168–174. https://doi.org/10.4055/cios20079
Harada Y, Wevers HW, Cooke TD (1988) Distribution of bone strength in the proximal tibia. J Arthroplasty 3(2):167–175. https://doi.org/10.1016/s0883-5403(88)80082-2
Tsukada S, Wakui M, Matsueda M (2013) Metal block augmentation for bone defects of the medial tibia during primary total knee arthroplasty. J Orthop Surg Res 8:36. https://doi.org/10.1186/1749-799X-8-36
Lee JK, Choi CH (2011) Management of tibial bone defects with metal augmentation in primary total knee replacement: a minimum five-year review. J Bone Joint Surg Br 93(11):1493–1496. https://doi.org/10.1302/0301-620x.93b10.27136
Obesity: preventing and managing the global epidemic. Report of a WHO consultation (2000). World Health Organ Tech Rep Ser 894, 1–253
Insall JN, Dorr LD, Scott RD, Scott WN (1989) Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 248:13–14
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Abdullah M, Selvanathan N, Kassim S, Kassim A, Chopra S (2020) Alternative Management Of Bone Defect In Primary Total Knee Replacement. Orthopaedic Journal of Sports Medicine 8 (5_suppl5):2325967120S2325900101
Mullaji AB, Padmanabhan V, Jindal G (2005) Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty 20(5):550–561. https://doi.org/10.1016/j.arth.2005.04.009
Ahmed I, Logan M, Alipour F, Dashti H, Hadden WA (2008) Autogenous bone grafting of uncontained bony defects of tibia during total knee arthroplasty a 10-year follow up. J Arthroplasty 23(5):744–750. https://doi.org/10.1016/j.arth.2007.08.021
Kharbanda Y, Sharma M (2014) Autograft reconstructions for bone defects in primary total knee replacement in severe varus knees. Indian J Orthop 48(3):313–318. https://doi.org/10.4103/0019-5413.132525
Completo A, Simoes JA, Fonseca F, Oliveira M (2008) The influence of different tibial stem designs in load sharing and stability at the cement-bone interface in revision TKA. Knee 15(3):227–232. https://doi.org/10.1016/j.knee.2008.01.008
Arslan A (2018) Using Tibia Proximal Cut Autograft in Advanced Varus Knee Deformity in Total Knee Arthroplasty; Outcomes Compared to the Control Group. Open Orthop J 12:405–410. https://doi.org/10.2174/1874325001812010405
Baek SW, Kim CW, Choi CH (2013) Management of tibial bony defect with metal block in primary total knee replacement arthroplasty. Knee Surg Relat Res 25(1):7–12. https://doi.org/10.5792/ksrr.2013.25.1.7
Tang Q, Guo S, Deng W, Zhou Y (2023) Using novel porous metal pillars for tibial bone defects in primary total knee arthroplasty. BMC Musculoskelet Disord 24(1):829. https://doi.org/10.1186/s12891-023-06962-1
Hamai S, Miyahara H, Esaki Y, Hirata G, Terada K, Kobara N, Miyazaki K, Senju T, Iwamoto Y (2015) Mid-term clinical results of primary total knee arthroplasty using metal block augmentation and stem extension in patients with rheumatoid arthritis. BMC Musculoskelet Disord 16:225. https://doi.org/10.1186/s12891-015-0689-9
Whittaker JP, Dharmarajan R, Toms AD (2008) The management of bone loss in revision total knee replacement. J Bone Joint Surg Br 90(8):981–987. https://doi.org/10.1302/0301-620X.90B8.19948
Dorr LD, Ranawat CS, Sculco TA, McKaskill B, Orisek BS (2006) Bone graft for tibial defects in total knee arthroplasty. Clin Orthop Relat Res 446:4–9. https://doi.org/10.1097/01.blo.0000214430.19033.b3
Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noel L, Strong DM (2012) Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project NOTIFY. Int Orthop 36(3):633–641. https://doi.org/10.1007/s00264-011-1391-7
Watanabe W, Sato K, Itoi E (2001) Autologous bone grafting without screw fixation for tibial defects in total knee arthroplasty. J Orthop Sci 6(6):481–486. https://doi.org/10.1007/s007760100001
Hosaka K, Saito S, Oyama T, Fujimaki H, Cho E, Ishigaki K, Tokuhashi Y (2017) Union, knee alignment, and clinical outcomes of patients treated with autologous bone grafting for medial tibial defects in primary total knee arthroplasty. Orthopedics 40(4):e604–e608. https://doi.org/10.3928/01477447-20170418-01
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No financial support was received for this study.
Author information
Authors and Affiliations
Contributions
A.A.D designed the study, performed the surgeries. M.K.M did analysis and interpretation of data and preliminary manuscript preparation. M.M.M. and A.M.E. contributed to the study design and final manuscript revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current study was approved by Menoufia University Institutional Review Board (IRB). Informed consent to participate in this study was obtained from patients.
Consent for publication
Consent to publish individual data was obtained from patients.
Competing interests
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dewidar, A.AM., Mesregah, M.K., Mesriga, M.M. et al. Autogenous structural bone graft reconstruction of ≥ 10-mm-deep uncontained medial proximal tibial defects in primary total knee arthroplasty. J Orthop Traumatol 25, 22 (2024). https://doi.org/10.1186/s10195-024-00762-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-024-00762-6