Abstract
Background
Surgical site infections (SSI) are the most frequent early complications of hand surgeries. However, the indications still remain uncertain for antibiotic prophylaxis in elective clean soft tissue surgeries of the hand and upper limb. Therefore, a systematic review of the literature and a meta-analysis was conducted to investigate the impact of antibiotic prophylaxis on the prevention of SSI in these types of surgeries.
Methods
An electronic search was performed in the following databases: MEDLINE/Pubmed, PMC/Pubmed, Web of Science/Clarivate Analytics, Embase/Elsevier, Scopus/Elsevier, BVS/Lilacs, and the Cochrane Library, with no restrictions regarding publication language or date. The primary outcome of interest was the occurrence of SSI following elective clean soft tissue surgeries of the hand and upper limb according to the administration of preoperative antibiotic prophylaxis and no antibiotic prophylaxis. Surgeries involving simultaneous bone procedures or orthopedic implants were excluded. Study selection and data extraction were conducted independently by two reviewers. RoB 2.0 and ROBINS-I are Cochrane risk-of-bias tool for randomized trials and non-randomized studies of interventions. The magnitude of the intervention effect was estimated using the relative risk (RR). The meta-analysis was performed with the Review Manager and R software tools, using the Mantel–Haenszel random-effects model and a 95% confidence interval (CI). Results with p ≤ 0.05 were considered statistically significant. The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.
Results
The initial search yielded 1175 titles, from which 12 articles met the inclusion criteria for the systematic review, and 10 were included in the subsequent meta-analysis. The majority of these studies were nonrandomized intervention trials, exhibiting a moderate risk of bias. According to our review, preoperative antibiotic prophylaxis did not have a statistically significant impact on the incidence of SSI (RR = 1.13, 95% CI 0.91–1.40, p = 0.28). The overall quality of evidence for this outcome was rated as low. Moderate statistical heterogeneity was observed (I2 = 44%), and the prespecified sensitivity analysis highlighted the consistency of the results.
Conclusions
While these results were consistent with the findings from individual studies included in this review, it is important to note that, given the threshold of p ≤ 0.05 for statistical significance, no definitive conclusions can be drawn from the quantitative analysis of the data obtained.
Level of evidence: Level 2.
Trial registration: CRD42023417786.
Similar content being viewed by others
Introduction
Surgical site infections (SSI) are the most frequent early complications of elective soft tissue hand surgeries. Despite millions of these surgical procedures being performed each year, these infections are rare, with rates between 0.3% and 1.5%, and are predominantly superficial [1,2,3,4].
Measures to control and prevent these outcomes include, among others, antibiotic prophylaxis. There is evidentiary support for the use of preoperative antibiotic prophylaxis for many orthopedic procedures (e.g., open fractures, lower-extremity fractures, and total joint replacement), but not for elective soft tissue hand surgical procedures [1, 3].
In this context, a recent international study with members of the American Society of Surgeons of the Hand showed that around half of the surgeons did not prescribe prophylactic antibiotics for the surgical treatment of carpal tunnel syndrome [5]. Likewise, an interview with members of the British Society for Surgery of the Hand found that around 80% of surgeons did not prescribe them for the surgical treatment of Dupuytren’s disease [6]. Prior to surgery, 13.6% (2009e2015) of patients received prophylactic intravenous antibiotics and trend analysis showed a statistically significant increase from 2009 (10.6%) to 2015 (18.3%), an increase of 72.5% [7].
However, observational, nonrandomized, nonblinded, single-center studies and, mainly, with a statistical power compromised by a sample size that is not large enough and representative of the investigated effect, have prevented the development of specific guidelines for careful antibiotic prophylaxis in these surgeries [8, 9]. As a result, decisions about the administration of preoperative antibiotic prophylaxis in elective clean soft tissue surgeries of the hand and upper limb are still based on the institution’s traditions and the surgeon’s preferences [1, 5, 6, 9, 10].
Therefore, this study aimed to investigate, through a systematic literature review and a meta-analysis, the impact of preoperative antibiotic prophylaxis on the prevention of SSI in this class of surgeries.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement, published in 2020, as demonstrated in additional file 1 (“PRISMA 2020 Checklist”) [11]. The study question was developed using the PICO acronym, where “P” represents the study population (patients submitted to elective clean soft tissue surgeries of the hand and upper limb), “I” defines the intervention to be investigated (administration of preoperative prophylactic antibiotics), “C” refers to the comparison of treatments (administration of placebo or no antibiotic prophylaxis), and “O” refers to the outcome investigated (occurrence of SSI). Therefore, the study question was: “Does preoperative antibiotic prophylaxis in elective clean soft tissue surgeries of the hand and upper limb prevent SSI?.
A systematic electronic search was performed in April 2023 in the following databases: MEDLINE/Pubmed, PMC/Pubmed, Web of Science/Clarivate Analytics, Embase/Elsevier, Scopus/Elsevier, BVS/Lilacs, and the Cochrane Library, using the search strategy that was built and validated with the collaboration of a librarian from the School of Medical Sciences at UNICAMP: (“Antibiotic Prophylaxis” OR Premedication) AND ((Hand AND “Upper Extremity”) OR Hand) AND “General Surgery” AND (“Postoperative Complications” OR “Surgical Wound Infection”), as described in additional file 2 (“Search strategies and information sources”). As “soft tissue” did not enable to retrieve relevant articles in preliminary searches, this term was discarded. The study protocol is available in the Prospective Register of Systematic Reviews (PROSPERO) international database under code CRD42023417786.
Our review included articles published in any period, language, or country, whose human adult or pediatric patients had undergone elective clean soft tissue surgeries of the hand and upper limb and whose primary outcome—incidence of SSI after this class of surgeries—had been described with the administration of preoperative antibiotic prophylaxis (any antimicrobial or dosage used) and without antibiotic prophylaxis.
The following articles were excluded: (1) studies that did not discriminate soft tissue surgeries from those involving simultaneous bone procedures or orthopedic implants, or clean surgeries from those in which patients had history of previous local infection; (2) studies whose postoperative follow-up was less than 4 weeks; (3) studies performed with animals or in vitro studies; (4) literature reviews, systematic reviews and meta-analyses, case series and reports, book chapters, letters, expert comments or opinions, expert panel, consensus statements, editorials, interviews, seminars, posters; and (5) unpublished or incomplete articles or articles that did not provide enough data to define the eligible population or assess the primary outcome.
Study selection and data extraction were conducted independently by two reviewers (GAN, MAC), according to predefined eligibility criteria. Disagreements were resolved by consensus among reviewers or arbitration by a senior reviewer (MFMA).
Selected articles had their full texts revised and data extracted into an especially developed form containing the variables of interest: main author, year of publication, country, conflict of interests, funding sources, study design, follow-up, surgical procedures performed, demographic characteristics of the population and potential risk factors for SSI, sample size, absolute number of participants who met the eligibility criteria of this systematic review, absolute number of patients who received preoperative antibiotic prophylaxis (case group), absolute number of patients who received placebo or no drug prophylaxis (control group), absolute number of cases that evolved to SSI in each group, absolute number of cases with severe surgical site infections, i.e., that required a new surgical approach or hospitalization for infection treatment, occurrence of minor complications of wounds, occurrence of adverse reactions and side effects related to antimicrobials, and information about costs when comparing interventions.
Two independent reviewers (GAN, ACAJ) assessed the risk of bias using the Cochrane tools RoB 2.0 and ROBINS-I for randomized trials and nonrandomized intervention studies, respectively [12, 13]. Finally, the quality of evidence was classified according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.
Meta-analysis
Statistical analyses were performed using the Review Manager and R software tools. The magnitude of the intervention effect was estimated by relative risk (RR) and the Mantel–Haenszel random-effects model was used in the meta-analysis. A 95% confidence interval (CI) was adopted and p ≤ 0.05 was considered statistically significant. Heterogeneity was assessed through visual inspection of forest plots and Cochran’s Q-tests (p ≤ 0.1), I2, and Tau2, and the recognition of significant heterogeneity would lead to verification of collected data, exclusion of relevant outliers, and primary studies with inconsistent methodological characteristics, as well as comparison of results obtained using meta-analyses of fixed and random effects. Finally, publication bias was assessed by visual inspection of the funnel plot and Egger’s test (p ≤ 0.05).
Results
Review statistics
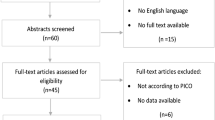
The initial search strategy found 1175 articles; of these, 555 were removed due to duplicates. After reading this titles and abstracts, 44 were selected for full-text review. Of these, 18 were excluded for not meeting the eligibility criteria and 4 because they were not fully available, as detailed in additional file 3 (“Reports excluded”). Therefore, 12 articles were included in this systematic review and 10 in the meta-analysis, two of which were excluded (Hoel et al. and Wachtel et al.) for presenting a number of events equal to zero in at least one of the comparison groups [4, 7, 14,15,16,17,18,19,20,21,22,23] (Fig. 1).
Study quality assessment
Eligible articles addressed the most common elective clean soft tissue surgeries of the hand and upper limb in clinical practice (e.g., open and endoscopic carpal tunnel release and carpal tunnel release revision, trigger finger release, cubital tunnel release, ulnar nerve transposition at the elbow, release of the ulnar nerve at the wrist, first extensor compartment release, fasciectomy for palmar fibromatosis and Dupuytren contracture release, tumor excision, tenosynovectomy of flexor tendons, fasciotomy, soft tissue laceration, tendon injury, nerve injury and/or vessel injury, tendon transfer, and wrist soft tissue arthroscopy) and had a combined population sample of 817,805 participants. Cefazolin was the antimicrobial used in prophylaxis of most studies, except in cases of previous reactions to cephalosporin or penicillin, when clindamycin was used instead [7, 14, 15, 17, 20,21,22,23]. The minimum postoperative follow-up time was 30 days in all studies and the diagnostic criteria can be considered more or less homogeneous [4, 7, 16,17,18,19,20,21,22,23]. Tables 1 and 2 summarize the main characteristics of the primary studies included in our review and each of the comparison groups.
A rate of 0.3–3.64% of SSI was observed after this class of surgeries in selected primary studies, and no statistical difference was observed in the incidence of infections with the administration of preoperative antibiotic prophylaxis [4, 7, 16,17,18,19,20,21,22,23]. Tables 3 and 4 show serious complications resulting from SSI, such as required surgical re-treatment and/or hospitalization, and possible adverse reactions and side effects related to the use of antimicrobials.
One of these studies was a randomized double-blind, placebo-controlled clinical trial, with low risk of bias in RoB 2.0, as illustrated in Fig. 2 [14]. Another one was a prospective intervention study whose patients were categorized into groups according to the institution where they were admitted, observing a moderate risk of bias in ROBIS-I mainly due to the lack of secrecy regarding the intervention, with possible bias in outcome evaluation [15]. The other studies included were observational cohort studies, all of them presenting a moderate risk of bias in the ROBIS-I, as illustrated in Figs. 3 and 4 [4, 7, 16,17,18,19,20,21,22,23]. Of these, the studies by Johnson et al. and Li et al. accounted for 98.14% of participants in our systematic review and were conducted using databases of medical claims and, therefore, have methodological limitations attributed to reliance on adequate coding and lack of access to medical records and patients [7, 18].
Tosti et al. did not declare any conflicts of interest while conducting their study [20]. Harness et al. received funding from the Kaiser Foundation Health Plan and Zheng et al. from the National Institutes of Health [4, 23]. The authors of the other articles declared no potential conflicts of interest regarding their studies, authorship, and/or article publication, and that no funding was received for their studies, authorship, and/or article publication. [4, 7, 14,15,16,17,18,19,20,21,22,23]
Meta-analysis
In the quantitative data analysis, the prescription of preoperative prophylactic antibiotics did not have a statistically significant effect on the prevention of SSI when compared with the administration of placebo or no antibiotic prophylaxis (RR = 1.13; 95% CI 0.91–1.39; Z = 1.1; p = 0.27) (Fig. 5).
Statistical heterogeneity was considered moderate (chi-squared = 16.01, degrees of freedom (df) = 9, p = 0.07; I2 = 44%; Tau2 = 0.03), so the prespecified sensitivity analysis was conducted (Fig. 5). Collected data were assessed by two independent reviewers. Studies assessing databases of medical claims were excluded from the meta-analysis, observing partial overlapping of confidence intervals and more or less similar effect estimates in the forest plot, as well as results that are consistent with those of the initial meta-analysis (Fig. 6) [6, 7]. Also, meta-analyses using fixed and random effects models had the same conclusions (Fig. 7). Therefore, despite the moderate statistical heterogeneity, the evidence found in our analysis was consistent.
Finally, publication bias was assessed by visual inspection of funnel plots, and no asymmetry was observed suggesting that studies with small samples and unfavorable results had not been disclosed (Fig. 8). Likewise, Eggers’s linear regression test conducted in R software confirmed this hypothesis (t = 0.97, df = 8, p = 0.36).
Evidence of effectiveness
Most studies included cannot be considered comparable to a well-planned randomized clinical trial, indicating some problems that must be considered when interpreting the results. Also, despite a population sample of considerable size (n = 817,309), the number of diagnosed events (n = 8734), and a narrow 95% CI, the results included RR = 1. As a result, confidence in the pooled effect estimates was reduced for two reasons—methodological limitations and imprecision—and the quality of evidence was considered low in the GRADE assessment (Table 5).
Explanation
-
a.
Twelve studies were included in the systematic review; of these, ten were included in the meta-analysis and two studies found no patient with SSI in the case and/or control groups.
-
b.
One out of ten studies included in the systematic review was a randomized, double-blind, placebo-controlled clinical trial; nine out of ten studies, on the other hand, were nonrandomized intervention studies, which together represented a weight of 96% in the meta-analysis.
-
c.
One out of ten studies included in the meta-analysis had a low risk of bias in the RoB 2.0 tool and nine out of ten studies had a moderate risk of bias in the ROBIS-I tool, mainly due to nonblinded measurement of the outcome by potentially biased raters.
-
d.
The forest plot showed partial overlapping of the confidence intervals of the studies, which are more or less similar results. Also, the statistical analyses showed chi-squared = 16.01 (df = 9; p = 0.07), I2 = 44%, and Tau2 = 0.03. The prespecified sensitivity analysis showed that heterogeneity did not impact the results.
-
e.
Although one out of ten studies excludes patients with risk factors for the occurrence of SSI, such as immunosuppression and other comorbidities, and one out of ten studies has the diagnosis of SSI inferred by the use of oral antibiotics in the postoperative period or the need for surgical re-approach, both accounted for only 10.6% weight in the meta-analysis. Likewise, these exclusions from the meta-analysis did not impact the results.
-
f.
Despite the high number of participants (n = 817,309), the number of events (n = 8734), and narrow 95% confidence interval (0.91–1.40), the pooled effects estimates included RR = 1 and values compatible with reduction, increase, and also absence of effect, resulting in result uncertainty.
-
g.
SSIs were considered serious when their treatment demanded new surgical procedures and/or hospitalization.
Selection bias
We believe no bias was present in the review process, as we used a comprehensive search strategy, including observational studies, as we knew beforehand the rarity of randomized, double-blind, controlled clinical trials that could answer the study question. In addition, data about the primary outcome were easily extracted from the primary studies included in our review.
Discussion
It is unclear whether preoperative antibiotics are necessary for elective clean hand and upper limb surgeries. The dilemma lies in the potential benefits of preventing surgical site infections versus the associated risks of its use. Problems associated with the excessive use of antibiotics include an increase in bacterial resistance with consequent reduction in the overall efficacy of these drugs, risk of adverse reactions and side effects, anaphylactic shock, infections by Clostridium difficile, and delayed wound healing. This uncertainty puts a strain on healthcare resources in terms of personnel and finances [9, 10, 23,24,25,26].
When it comes to evaluating the effectiveness of health interventions, randomized clinical trials are considered the best study design. However, there are limited studies in scientific literature that specifically explore the use of antibiotics to prevent surgical site infections in elective clean hand and upper limb surgeries. Moreover, there are very few studies with enough sample sizes to produce reliable, statistically significant results. The fact is that, although at the top of the evidence pyramid, some questions are unlikely to be answered by authors using randomized clinical trials, and this is probably one of these questions [8].
While there are narrative reviews available on this subject, they do not make distinctions between elective and nonelective surgeries, or between procedures that solely involve soft tissue and those that include concurrent bone procedures or the placement of orthopedic implants [9, 10]. However, the decision about whether or not to prescribe prophylactic antibiotics is made every time this type of surgery is performed.
In our review, only 0.2% of patients (n = 24/14,255) demanded new surgical procedures and/or hospitalization related to SSI [4, 15, 16, 19,20,21,22,23]. Also, the prescription of preoperative prophylactic antibiotics had no impact on the incidence of SSI when compared with the administration of placebo or no prophylaxis (RR = 1.13; 95% CI 0.91–1.40; z = 1.1; p = 0.28). However, although this result is aligned with the evidence observed in the primary studies selected for this review, considering p ≤ 0.05 as statistically significant, no conclusion can be reached from our data meta-analysis.
Bykowski et al., in a single-center retrospective analysis of 8850 elective hand surgery cases, using a multivariate regression analysis, concluded that diabetes mellitus (OR = 2.8, 95% CI 1.2–6.5, p = 2 × 10–2), smoking (OR = 3.0, 95% CI 1.5–6.2, p = 3 × 10–3), and longer surgical time (OR = 1.02, 95% CI 1.01–1.03, p = 1 × 10–4) are positive predictors of SSI regardless of the administration of antimicrobials [16]. Shapiro et al., in a critical analysis review, found that there is a paucity of literature evaluating the use of preoperative antibiotic prophylaxis in patients with rheumatoid arthritis, those with cardiac valves, and those taking corticosteroids. There are other well-known risk factors for the occurrence of infections in general, but the literature has no study specifically assessing the effect of antimicrobials on the prevention of SSI after elective clean soft tissue surgeries of the hand and upper limb in these populations [1].
Although we do not have reasonable evidence to answer these questions, some related facts are well established in the literature; for example, the potential harmful effects of general and universal antibiotic prophylaxis which is probably minimally effective in the prevention of SSI. In this context, Sandrowski et al. observed 1.5% of adverse reactions after the preoperative single-dose administration of antibiotics to a cohort of 551 patients undergoing outpatient surgeries of the hand and upper limb [24]. According to Wachtel et al., one out of ten patients who receive antimicrobials show adverse reactions (16.2% versus 5.5%; p = 0.03) [22]. Likewise, a recent review described rates of up to 0.1% anaphylaxis due to the administration of cephalexin, as well as 21% diarrhea, and up to 8% infection caused by Clostridium difficile after the administration of clindamycin [10]. Finally, Tacconelli et al., in a systematic review of the literature and meta-analysis of total 24,230 patients, observed that exposure to antibiotics almost doubles the risk of infection by methicillin-resistant Staphylococcus aureus (RR = 1.8, 95% CI 1.7–1.9, p < 0.001) [26].
In another perspective, if prophylactic antibiotics were not routinely administered, at least US $15–30 million would be saved every year in the USA [10]. In this regard, Johnson et al. noted that total healthcare expenditures in the first 30 days after surgery are higher in cases where preoperative intravenous antibiotics are administered when compared with cases that do not receive drug prophylaxis (US $6070 versus US $4891, respectively; p < 0.001) [7].
Study strengths and limitations
This study has strengths that should be highlighted. First, the PRISMA declaration guidelines were used for the development of a detailed protocol, externally reviewed and publicly registered on the international PROSPERO platform. A well-documented and sensitive search strategy enabled the retrieval of more than 1100 titles. Article selection and data extraction were performed independently by two reviewers, with disagreements arbitrated by a senior reviewer. The same procedure was used in risk of bias assessments for randomized clinical trials and nonrandomized intervention studies, as recommended by the RoB 2.0 and ROBIS-I tools, in this order. Finally, judgment and classification of the level of certainty of the evidence was performed using the structured, reproducible, and transparent approach defined in the GRADE system.
In addition to the judicious methodology, the size of the investigated population sample of 817,805 patients should also be highlighted. This large population would not probably be obtained if the studies were not combined. However, considering the general low incidence of SSI in the context of elective clean soft tissue surgeries of the hand and upper limb, a considerable population would be critical for the detection of a potentially small effect, with adequate statistical power, such as the one investigated in our review.
In contrast, our study has some limitations. First, information was retrieved from articles published in the literature and, therefore, from secondary sources. Then, data about some important developments related to antibiotic prophylaxis, for example, serious complications resulting from SSI, and adverse reactions and side effects to the use of these drugs, were not always available. Second, considering this is a systematic review, patients who met the eligibility criteria showed differences in their baseline characteristics. Also, the variables that influenced the decision of whether or not to use antibiotic prophylaxis were different among the included studies, as they depended on the surgeon’s personal experience and the tradition of the institution where the surgical procedure was performed, particularly when considering the absence of randomization and specific guidelines for antibiotic prophylaxis in elective clean soft tissue surgeries of the hand and upper limb. Finally, although the criteria for SSI diagnosis are documented and consistent with each other in the selected primary studies, no standardization was found in their definition and measurement of results, nor blinding of outcome raters regarding the group to which participants were allocated. Then, grouping of data potentially introduced confounding factors, which are inherent to systematic reviews of observational studies, although these studies remain valid and often the only feasible sources of information in the investigation of uncommon outcomes, such as SSI in this class of surgeries.
Conclusions
Implications for practice
Low-quality evidence suggests that there is no statistically significant difference between the use of preoperative antibiotic when compared with placebo or no drug prophylaxis for the prevention of SSI in elective clean soft tissue surgeries of the hand and upper limb. Thus, we believe that other perioperative prophylactic measures, such as hand washing, adequate skin preparation, and the use of surgical drapes and sterile technique, are more effective and less harmful than the administration of antimicrobials and therefore we discourage their use in this class of surgeries.
Implications for research
Controlled clinical trials with appropriate randomization and blinding methods and recruitment strategies that can ensure generalization of the results obtained would be the preferred study design to assess the real efficacy of antibiotic prophylaxis in the prevention of SSI after elective clean soft tissue surgeries of the hand and upper limb. However, given the infrequency of this outcome in this class of surgeries, these clinical trials would require a population sample of thousands of participants or even more, for example, in cases including analyses of subgroups of patients with certain characteristics that make them susceptible to infections.
However, if this is not feasible, an alternative would be to conduct studies with large multicenter prospective cohorts. This would require an acceptable rate of clinically relevant SSI in terms of use of human, technical, and financial healthcare resources versus the occurrence of complications and sequelae secondary to these infections, with these prospective studies being fed until a statistically significant difference could be detected between the comparison groups. However, we may already be within an acceptable rate of SSI in this class of surgeries only with nondrug prophylactic practices generally implemented today.
Even so, these studies assessing large, multicenter prospective cohorts could support the development of a probability calculator that provides a composite measure for the risk of infection according to the patient’s health status and the type of surgical procedure, guiding the indication of preoperative antibiotic prophylaxis on a case-by-case basis and enabling an informed and shared decision between physicians and their patients.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ABP:
-
Antibiotic prophylaxis
- df:
-
Degrees of freedom
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluation
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- M-H:
-
Mantel–Haenszel
- m2 :
-
Square meters
- kg:
-
Kilograms
- PL/∅:
-
Placebo or no drug prophylaxis
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- RR:
-
Relative risk
- SSI:
-
Surgical site infection
References
Shapiro LM, Zhuang T, Li K, Kamal RN (2019) The use of preoperative antibiotics in elective soft-tissue procedures in the hand: a critical analysis review. JBJS Rev 7(8):e6. https://doi.org/10.2106/JBJS.RVW.18.00168
Lipira AB, Sood RF, Tatman PD, Davis JI, Morrison SD, Ko JH (2015) Complications within 30 days of hand surgery: an analysis of 10,646 patients. J Hand Surg Am 40(9):1852–59.e3. https://doi.org/10.1016/j.jhsa.2015.06.103
Menendez ME, Lu N, Unizony S, Choi HK, Ring D (2015) Surgical site infection in hand surgery. Int Orthop 39(11):2191–2198. https://doi.org/10.1007/s00264-015-2849-9
Harness NG, Inacio MC, Pfeil FF, Paxton LW (2010) Rate of infection after carpal tunnel release surgery and effect of antibiotic prophylaxis. J Hand Surg Am 35(2):189–196. https://doi.org/10.1016/j.jhsa.2009.11.012
Munns JJ, Awan HM (2015) Trends in carpal tunnel surgery: an online survey of members of the American Society for Surgery of the Hand. J Hand Surg Am 40(4):767–71.e2. https://doi.org/10.1016/j.jhsa.2014.12.046
Kadhum M, Sinclair P, Middleton C (2020) The use of prophylactic antibiotics in surgery for Dupuytren’s disease: a survey of hand surgeons. World J Plast Surg 9(2):135–140. https://doi.org/10.29252/wjps.9.2.135
Johnson SP, Zhong L, Chung KC, Waljee JF (2018) Perioperative antibiotics for clean hand surgery: a national study. J Hand Surg Am 43(5):407-416.e1. https://doi.org/10.1016/j.jhsa.2017.11.018
Leopold SS (2018) Editor’s spotlight/take 5: effectiveness of preoperative antibiotics in preventing surgical site infection after common soft tissue procedures of the hand. Clin Orthop Relat Res 476(4):660–663. https://doi.org/10.1007/s11999.0000000000000231
Eberlin KR, Ring D (2015) Infection after hand surgery. Hand Clin 31(2):355–360. https://doi.org/10.1016/j.hcl.2014.12.007
Dunn JC, Fares AB, Kusnezov N et al (2018) Current evidence regarding routine antibiotic prophylaxis in hand surgery. Hand (NY) 13(3):259–263. https://doi.org/10.1177/1558944717701241
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Aydin N, Uraloğlu M, Burhanoğlu ADY, Sensöz Ö (2010) A prospective trial on the use of antibiotics in hand surgery. Plast Reconstr Surg 126(5):1617–1623. https://doi.org/10.1097/PRS.0b013e3181ef90cb
Bäcker HC, Freibott CE, Wilbur D et al (2021) Prospective analysis of hand infection rates in elective soft tissue procedures of the hand: the role of preoperative antibiotics. Hand (N Y) 16(1):81–85. https://doi.org/10.1177/1558944719842238
Bykowski MR, Sivak WN, Cray J, Buterbaugh G, Imbriglia JE, Lee WP (2011) Assessing the impact of antibiotic prophylaxis in outpatient elective hand surgery: a single-center, retrospective review of 8,850 cases. J Hand Surg Am 36(11):1741–1747. https://doi.org/10.1016/j.jhsa.2011.08.005
Hoel RJ, Mittelsteadt MJ, Samborski SA, Bohn DC (2018) Preoperative antibiotics in wrist arthroscopy. J Hand Surg Am 43(11):987-991.e1. https://doi.org/10.1016/j.jhsa.2018.03.040
Li K, Sambare TD, Jiang SY, Shearer EJ, Douglass NP, Kamal RN (2018) Effectiveness of preoperative antibiotics in preventing surgical site infection after common soft tissue procedures of the hand. Clin Orthop Relat Res 476(4):664–673. https://doi.org/10.1007/s11999.0000000000000073
Mehta S, Court T, Graf A, Best C, Havlik R (2022) The impact of clinical practice guidelines on preoperative antibiotic administration for carpal tunnel release. Hand (N Y) 18(5):780–784. https://doi.org/10.1177/15589447211063543
Tosti R, Fowler J, Dwyer J, Maltenfort M, Thoder JJ, Ilyas AM (2012) Is antibiotic prophylaxis necessary in elective soft tissue hand surgery? Orthopedics 35(6):e829–e833. https://doi.org/10.3928/01477447-20120525-20
Vasconcelos C, Serra M, Nogueira RM, Carmo LD (2017) Antibiotic prophylaxis in elective hand surgery. Revista Iberoamericana de Cirugía de la Mano 45:89–93. https://doi.org/10.1055/s-0037-1608625
Wachtel N, Meyer E, Volkmer E et al (2023) Efficacy of perioperative antibiotic prophylaxis in elective soft-tissue-only wrist arthroscopy. Bone Jt Open 4(4):219–225. https://doi.org/10.1302/2633-1462.44.BJO-2023-0019
Zheng A, Fowler JR (2022) The effectiveness of preoperative antibiotic prophylaxis in ulnar nerve release at the cubital tunnel. Hand (N Y). https://doi.org/10.1177/15589447221107688
Sandrowski K, Edelman D, Rivlin M et al (2020) A prospective evaluation of adverse reactions to single-dose intravenous antibiotic prophylaxis during outpatient hand surgery. Hand (N Y) 15(1):41–44. https://doi.org/10.1177/1558944718787264
Kemaloğlu CA, Günay GK, Perçin D, Deniz K (2014) An unpredicted side effect of prophylactic antibiotic use. J Chemother 26(3):154–158. https://doi.org/10.1179/1973947813Y.0000000131
Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R (2008) Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother 61(1):26–38. https://doi.org/10.1093/jac/dkm416
Acknowledgements
The authors thank Ana Paula de Morais e Oliveira for collaboration in the development and validation of the literature search strategies. The authors thank Espaço da Escrita—Pró-Reitoria de Pesquisa—UNICAMP – for the language services provided.
Funding
The authors received financial support for the publication of this article from Fundação CAPES/ Rachel Meneguello.
Author information
Authors and Affiliations
Contributions
Conceptualization, GAN, MFMA, RGP. Methodology, GAN, RGP. Data curation, GAN, RGP. Formal analysis, GAN, RGP. Investigation, GAN, ACAJ, MAC, MFMA. Validation, GAN, ACAJ, MAC, MFMA, RGP, SA. Writing—original draft preparation, GAN. Writing—review and editing RGP,SA. Visualization, GAN, RGP, SA. Supervision, RGP, SA, MFMA. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work does not require approval by an ethics committee since it is a systematic review and meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors declared no potential competing interests with respect to the research, authorship, and publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1.
PRIMA checklist.
Additional file 2. Table S2.
Search strategies.
Additional file 3. Table S3.
Reports excluded.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Negri, G.A., Andrade Junior, A.C., Cox, M.A. et al. Preoperative antibiotic prophylaxis and the incidence of surgical site infections in elective clean soft tissue surgery of the hand and upper limb: a systematic review and meta-analysis. J Orthop Traumatol 25, 4 (2024). https://doi.org/10.1186/s10195-024-00748-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-024-00748-4