Abstract
Background
Multiple doses of dexamethasone and tranexamic acid can inhibit postoperative inflammation and reduce fibrinolysis and perioperative blood loss in total knee arthroplasty. In this single-center, double-blind, randomized clinical trial, the aim was to investigate whether applying a tourniquet to patients on dexamethasone and tranexamic acid could further reduce perioperative blood loss.
Materials and methods
Patients who underwent cemented total knee arthroplasty at our hospital were randomized to receive a tourniquet (n = 71) or not (n = 70) during the procedure. All patients received multiple doses of dexamethasone and tranexamic acid perioperatively. The primary outcome was perioperative blood loss, while secondary outcomes were surgery duration, postoperative laboratory indices of inflammation and fibrinolysis, range of knee motion, VAS pain score, knee circumference, knee swelling rate, homologous transfusion, albumin use, and complications.
Results
Using a tourniquet was associated with significantly lower intraoperative blood loss (P < 0.001) and total blood loss (P = 0.007) as well as significantly shorter surgery duration (P < 0.001). In contrast, the tourniquet did not significantly affect hidden blood loss, postoperative inflammation or fibrinolysis, range of knee motion, VAS pain score, knee circumference, knee swelling rate, homologous transfusion, albumin use, or complications.
Conclusions
The results of this randomized clinical trial demonstrate that applying a tourniquet during cemented total knee arthroplasty to patients receiving multiple doses of dexamethasone and tranexamic acid can further reduce perioperative blood loss without increasing the risk of inflammation, fibrinolysis, or other complications. Thus, it is advised to use tourniquets combined with dexamethasone and tranexamic acid to reduce perioperative blood loss and avoid tourniquet-related adverse events.
Level of evidence: Therapeutic Level I.
Trial registration Chinese Clinical Trail Registry, ChiCTR2200060567. Registered 5 June 2022—retrospectively registered, http://www.chictr.org.cn/showproj.aspx?proj=171291.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) is considered the most effective therapy for patients with end-stage knee arthritis because it can relieve pain and improve joint function [1]. However, TKA is usually accompanied by massive intraoperative bleeding and a poor surgical field of view, which may prolong surgery and inhibit cement penetration [2, 3]. Longer surgery increases the risk of surgical site infection, re-operation, and blood transfusion [4].
Applying tourniquets during surgery can maintain a clear surgical field by reducing intraoperative blood loss, thereby shortening the operation time. However, their use in orthopedic procedures such as TKA is controversial, given that they can increase the risk of postoperative thigh pain, limb swelling, nerve palsy, muscle injury, and deep vein thrombosis [2, 5, 6]. Tourniquets can also increase hidden blood loss, resulting in greater total blood loss [2, 5, 7].
We wondered whether the disadvantages of tourniquets would be mitigated by the perioperative dexamethasone typically used during TKA to inhibit postoperative inflammation, vomiting, and thigh pain [8,9,10,11]. In particular, multiple doses of dexamethasone can enhance postoperative recovery [9, 11, 12]. In addition to dexamethasone, TKA patients are often given tranexamic acid, which inhibits plasminogen activation to prevent fibrinolysis, thereby reducing hidden blood loss [13,14,15,16,17]. Numerous studies have shown that intravenous perioperative tranexamic acid can reduce postoperative blood loss and transfusion rates without increasing the risk of deep vein thrombosis or pulmonary embolism [18,19,20,21,22,23,24,25].
Here we tested the safety and efficacy of applying tourniquets to patients undergoing cemented TKA involving multiple doses of dexamethasone and tranexamic acid.
Materials and methods
Patients and randomization
This study has been reported in line with Consolidated Standards of Reporting Trials (CONSORT 2010) Guidelines. This single-center, double-blind, randomized controlled trial was approved by the Biomedical Ethics Committee of Sichuan University West China Hospital (date February 8, 2022/no. 2021–1699). All patients who were candidates for cemented TKA at our hospital from February 2022 to June 2022 were considered for inclusion. Patients were included if they underwent TKA for knee osteoarthritis in our hospital and showed a flexion-contracture deformity of < 20°, varus or valgus deformity of < 20° [26]. Each patient provided written informed consent before surgery.
Patients were excluded if they had a history of knee infection, had a level of hemoglobin of < 100 g/L or coagulopathy, were using anticoagulants or antiplatelet drugs, had a body mass index (BMI) of > 40 kg/m2, or refused to participate in the study.
Prior to TKA, patients were randomly assigned 1:1 to a group that received a tourniquet during TKA or to a group that did not. Random numbers were generated using a computer algorithm and sealed in opaque envelopes. Each patient was asked to select an envelope, inside which their group allocation was indicated. Observers who collected data after surgery were not involved in the surgery and were unaware of the group allocation.
Anesthesia and surgery
All patients in our study received general anesthesia involving induction with midazolam (0.02–0.03 mg/kg), propofol (1–2 mg/kg), sulfentanyl (0.3–0.5 μg/kg), and rocuronium (0.6–1.0 mg/kg), which were delivered by intravenous bolus injection. Exceptions were patients older than 60 years, who did not receive midazolam. Anesthesia was maintained through continuous intravenous infusion of remifentanil (0.1–0.2 μg/kg·min) and continuous inhalation of sevoflurane. Rocuronium was added every 40–60 min at 25–33% of the induction dose. Sulfentanyl (5ug) was added every hour.
Intraoperative blood pressure was recorded every 3 min using an electrocardiogram and an upper-arm sphygmomanometer. Intraoperative blood pressure was maintained at baseline in the tourniquet group, or at approximately 70% of baseline in the non-tourniquet group. The target blood pressure was achieved through intravenous injection of m-hydroxylamine, ephedrine, and nicardipine.
All patients received antibiotics at 0.5–2 h before surgery. At 10 min before surgery, all patients received intravenous dexamethasone (10 mg) and intravenous tranexamic acid (60 mg/kg). Previous studies have confirmed the efficacy and safety of a preoperative high-dose (60 mg/kg) combined with postoperative multiple-dose tranexamic acid sequential application regimen [27,28,29,30,31,32]. Immediately before surgery, a tourniquet was applied at the base of the thigh in the tourniquet group and inflated to 100 mmHg above baseline systolic pressure. All surgeries were conducted by the same team of surgeons at our hospital, who had more than 10 years of experience in total joint arthroplasty, and were performed using a standardized medial parapatellar approach. All patients received the same type of cemented posterior-stabilized prosthesis (DePuy Synthes, Johnson and Johnson, New Brunswick, USA). During surgery, intramedullary guides were used for femoral preparation and extramedullary guides for tibial preparation. No postoperative drain was used.
Postoperative management
All patients stopped using antibiotics within 24 h after surgery, and all received intravenous tranexamic acid (1 g) at 3, 6, 12, and 24 h after surgery. All patients received intravenous dexamethasone (10 mg) on postoperative day 1 and intravenous dexamethasone (5 mg) on postoperative day 2, and they began to receive oral prednisone (10 mg) from postoperative day 2 onward in order to control pain and reduce inflammation. All patients were required to start functional exercise as soon as they had recovered from anesthesia and to begin walking under pain control from postoperative day 1. Every patient received a lower-extremity pump and a subcutaneous injection of low-molecular-weight heparin (2000 IU) to prevent deep vein thrombosis from postoperative day 1. The patients were discharged on the third day after the operation if they had no signs of complications and could walk independently.
All patients underwent Doppler ultrasonography either immediately if they showed any sign of deep vein thrombosis or otherwise on postoperative day 2 or 3 and 14. Patients were scheduled for computed tomography angiography if they suddenly experienced chest discomfort or breathing difficulties, if they coughed up pink foamy sputum, or if they exhibited other symptoms suggestive of pulmonary embolism.
Blood transfusion was performed according to the guidelines of the Chinese Ministry of Health [33]: transfusions were given to patients whose hemoglobin level was lower than 70 g/L and who did not present clinical symptoms or to those whose hemoglobin level was lower than 100 g/L and who presented anemia-related organ dysfunction, intolerable anemia symptoms, or ongoing hidden blood loss. Albumin was used for patients whose albumin level was less than 35 g/L for 2 consecutive days after surgery. Twenty grams of albumin were used each time and albumin levels were rechecked. Whether to use additional albumin was determined according to the results of the re-examination.
Data extraction and outcomes
Two investigators independently collected the following information from each patient: (1) basic information such as age, sex, height, weight, BMI, and comorbidities; (2) postoperative length of stay and overall length of hospitalization; (3) perioperative laboratory values, including pre- and postoperative hematocrit (Hct), hemoglobin (Hb), C-reactive protein (CRP), interleukin-6 (IL-6), fibrin degradation product (FDP), and D-dimer; (4) perioperative range of knee motion (ROM), knee circumference, and knee swelling rate; (5) intraoperative systolic blood pressure and blood loss; (6) postoperative VAS pain score; and (7) complications.
The primary outcome was perioperative blood loss, comprising total blood loss (TBL), intraoperative blood loss (IBL), and hidden blood loss (HBL). TBL was calculated using the Gross formula [34]:
where PBV is the predictive blood volume; Hctpre is the preoperative Hct level; Hctpost is the lowest postoperative Hct level, which usually occurred on postoperative day 2 or 3; and Hctavg is the average of Hctpre and Hctpost. PBV was calculated using the formula [35]
where k1 = 0.3669, k2 = 0.03219, k3 = 0.6041 for men, or k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for women. HBL was defined as the difference between TBL and IBL.
Secondary outcomes were surgery duration, postoperative laboratory indices of inflammation and fibrinolysis, range of knee motion, VAS pain score, knee circumference, knee swelling rate, homologous transfusion, albumin use, and complications. Complications included postoperative hypertension, deep vein thrombosis, pulmonary embolism, calf muscular venous thrombosis, aseptic or septic wound complications, periprosthetic joint infection, 30-day mortality, and 90-day readmission. Postoperative hypertension was defined as a systolic pressure of > 160 mmHg within 2 h after surgery as determined by electrocardiography and an upper-arm sphygmomanometer in the ward [36]. Systolic pressure was also recorded postoperatively using electrocardiography in the ward. Wound complications were defined as the need for intervention, such as superficial surgical debridement, re-suture, or a longer hospital stay [37]. If the wound showed secretion, at least two samples were cultured to test for the presence of bacteria. If two cultures were positive for homogeneous bacteria, wound complications were classified as septic [37]. Otherwise, wound complications were classified as aseptic [37]. The knee circumference was measured at the thigh at a position 10 cm above the upper edge of the patella when the patient was in a supine position with the knee straight. Knee swelling rate was calculated using the formula
where Cpre is the preoperative knee circumference and Cpost is the postoperative knee circumference measured on postoperative days 1, 2, and 3.
Statistical analysis
The minimum sample size was estimated based on a previous study in our institute [38]. In previous studies, the average duration of surgery without a tourniquet was 84.9 min with a standard deviation of 20.1 min. We assume that 10 min is the least clinically significant reduction in duration of surgery due to tourniquet application. The test power (1 − β) was 0.8 and the alpha error rate was 0.05. The lost-to-follow-up rate was set at 0.05. Calculations indicated that at least 68 patients were required for each group.
Statistical analysis was performed using SPSS 22.0 (IBM, Armonk, NY, USA). Continuous data with a normal distribution were expressed as mean ± standard deviation (SD), while categorical data were expressed as frequencies. Inter-group differences were analyzed for significance using the Mann–Whitney U test in the case of continuous data that were skewed or showed unequal variance, or using the independent samples t-test in the case of normally distributed continuous data. Inter-group differences in categorical data were assessed using the chi-squared test or Fisher’s exact test as appropriate. Differences with P < 0.05 were considered significant.
Results
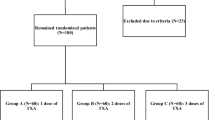
In this study, 150 patients were candidates for cemented TKA at our hospital. Six patients refused to participate in the study. Two patients were excluded due to coagulopathy and 1 patient was excluded due to severe anemia. We finally enrolled 141 patients in our study, allocating 71 patients to the tourniquet group and 70 to the non-tourniquet group (Fig. 1). All patients were followed up for at least 3 months. The two groups did not differ significantly in age, sex distribution, comorbidities, ROM, knee circumference, or preoperative values for Hb, Hct, CRP, IL-6, FDP, or D-dimer (Table 1). Similarly, they did not differ significantly in postoperative stay or overall hospital stay.
The use of a tourniquet was associated with significantly higher intraoperative systolic pressure and a shorter surgery (Table 2). It was also associated with significantly lower IBL and TBL (Fig. 2). In contrast, the use of a tourniquet did not significantly affect HBL, postoperative ROM, or the levels of CRP, IL-6, FDP, or D-dimer on postoperative days 1, 2, 3, or 14 (Table 3). Moreover, the use of a tourniquet did not significantly increase the postoperative VAS pain score, knee circumference, or knee swelling rate (Table 4).
In both groups, CRP levels peaked on postoperative day 3, while IL-6 levels peaked on postoperative day 2. Levels of both FDP and D-dimer peaked on postoperative day 14 (Fig. 3).
The two groups did not show significant differences in postoperative hypertension, deep vein thrombosis, pulmonary embolism, calf muscular venous thrombosis, septic or aseptic wound complications, 90-day readmission, or albumin use (Table 5). No patients died within 30 days, had a periprosthetic joint infection, or required homologous transfusion during follow-up.
Discussions
The most important finding of our study was that applying a tourniquet to patients undergoing cemented TKA involving multiple doses of dexamethasone and tranexamic acid reduced IBL without increasing HBL or the risk of complications. It also significantly shortened surgery and reduced intraoperative controlled hypotension requirements.
Several studies have shown that tourniquets can reduce IBL and thereby help ensure a bloodless surgical field in orthopedic procedures such as TKA [39,40,41]. Indeed, the bloodless surgical field may reduce the time needed to ensure hemostasis and identify structures during the procedure, which may help explain why the tourniquet shortened surgery in our study. The concepts of minimally invasive surgery and fine operation are related to careful hemostasis. Intraoperative trauma was minimized and an unnecessary release and synovectomy were not performed. For patients who use a tourniquet, every wound bleeding point should be completely electrocoagulated to stop the bleeding. Otherwise, obvious bleeding may occur after the tourniquet is loosened. Tourniquets have also been shown to significantly reduce TBL [42, 43]. Our results are consistent with that literature. Reducing IBL in TKA has been reported to improve cement penetration and initial fixation strength, reducing the long-term risk of aseptic loosening [3]. Whether tourniquets affect this is unclear, and we could not address this question because of the relatively short follow-up.
In addition, we found that tourniquet use did not increase HBL, in contrast to previous work [2, 5, 7]. The increase in HBL due to tourniquets has been attributed to the exacerbation of hyperfibrinolysis caused by surgical trauma, which could lead to knee swelling and pain [23, 44]. Thus, the key to decreasing the TBL due to tourniquets is to inhibit postoperative hyperfibrinolysis. Tranexamic acid is an anti-fibrinolytic agent that has been proven to inhibit plasminogen activation and reduce hidden blood loss [13, 18,19,20,21,22,23]. Using a tourniquet with intravenous tranexamic acid did not increase the levels of FDP and D-dimer after surgery, nor did they significantly increase hidden blood loss, knee swelling, or pain.
Like a tourniquet, hypotension anesthesia during TKA has been reported to provide a clear surgical field as well as reduce HBL and the risk of deep vein thrombosis [45,46,47]. On the other hand, it may increase the risk of postoperative acute kidney injury and myocardial damage, with the severity of damage being proportional to the extent and duration of hypotension [48, 49]. Applying a tourniquet may avoid these problems because, as we found here, it was associated with higher intraoperative systolic blood pressure. Another advantage of tourniquets is that they are more straightforward and less demanding to apply than hypotension anesthesia.
Applying tourniquets during TKA has been shown to increase the levels of the inflammatory factors CRP and IL-6, probably as a result of ischemia and damage to soft tissue [5]. Furthermore, the inflammatory response after TKA causes severe pain and postoperative nausea and vomiting, leading to delayed early rehabilitation and hospital discharge [9,10,11]. However, we found that tourniquets did not significantly alter the changes in the levels of CRP or IL-6 during TKA, or the VAS pain score or knee diameter. This may reflect that all patients received multiple doses of dexamethasone, which can effectively inhibit postoperative inflammatory responses, as reported by Xu et al. [9]. Tourniquet use combined with perioperative dexamethasone can reduce tourniquet-related inflammatory responses, promote postoperative recovery, and shorten the postoperative length of stay.
Xie et al. have reported that using a tourniquet could increase the incidence of postoperative deep vein thrombosis [42]. Zhou et al. demonstrated that high pressure and prolonged ligation lead to limb ischemia, and when the tourniquet is relieved, ischemia–reperfusion leads to secondary endothelial injury and thrombus formation [50]. Using tourniquets causes lower-limb ischemia and releases inflammatory cytokines to promote thrombus formation [51]. Our insistence on early ambulation after TKA and suppressing inflammation with multiple doses of dexamethasone may have also helped reduce the risk of thrombosis.
Our results should be verified and extended in studies with a longer follow-up in order to detect delayed or long-term complications. Future work with a longer follow-up should examine the safety and efficacy of tourniquets for patients undergoing cemented TKA involving multiple doses of dexamethasone and tranexamic acid, as well as the effects of tourniquets on the speed and extent of joint function recovery, based on the repeated measurement of the postoperative ROM. Ultimately, our findings should be validated with large multicenter studies.
Conclusions
Our randomized trial suggests that applying tourniquets to patients undergoing TKA involving multiple doses of dexamethasone and tranexamic acid can reduce perioperative blood loss without increasing HBL, postoperative inflammation, or risk of thrombosis, even though these have been described as adverse effects of tourniquets in orthopedic procedures such as TKA.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TKA:
-
Total knee arthroplasty
- VAS:
-
Visual analog scale
- BMI:
-
Body mass index
- Hct:
-
Hematocrit
- Hb:
-
Hemoglobin
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- FDP:
-
Fibrin degradation product
- ROM:
-
Range of knee motion
- TBL:
-
Total blood loss
- IBL:
-
Intraoperative blood loss
- HBL:
-
Hidden blood loss
- COPD:
-
Chronic obstructive pulmonary disease
References
Liddle AD, Pegg EC, Pandit H (2013) Knee replacement for osteoarthritis. Maturitas 75:131–136
Tai T-W, Lin C-J, Jou IM et al (2011) Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 19:1121–1130
Hegde V, Bracey DN, Johnson RM et al (2021) Tourniquet use improves cement penetration and reduces radiolucent line progression at 5 years after total knee arthroplasty. J Arthroplasty 36:S209–S214
Ahmed I, Chawla A, Underwood M et al (2021) Time to reconsider the routine use of tourniquets in total knee arthroplasty surgery. Bone Joint J 103:830–839
Zhao H-Y, Yeersheng R, Kang X-W et al (2020) The effect of tourniquet uses on total blood loss, early function, and pain after primary total knee arthroplasty: a prospective, randomized controlled trial. Bone Joint Res 9:322–332
Horlocker TT, Hebl JR, Gali B et al (2006) Anesthetic, patient, and surgical risk factors for neurologic complications after prolonged total tourniquet time during total knee arthroplasty. Anesth Analg 102:950–955
Schnettler T, Papillon N, Rees H (2017) Use of a tourniquet in total knee arthroplasty causes a paradoxical increase in total blood loss. J Bone Joint Surg Am 99:1331–1336
Gilron I (2004) Corticosteroids in postoperative pain management: future research directions for a multifaceted therapy. Acta Anaesthesiol Scand 48:1221–1222
Xu B, Ma J, Huang Q et al (2018) Two doses of low-dose perioperative dexamethasone improve the clinical outcome after total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc 26:1549–1556
Li D, Wang C, Yang Z et al (2018) Effect of intravenous corticosteroids on pain management and early rehabilitation in patients undergoing total knee or hip arthroplasty: a meta-analysis of randomized controlled trials. Pain Pract 18:487–499
Li D, Zhao J, Yang Z et al (2019) Multiple low doses of intravenous corticosteroids to improve early rehabilitation in total knee arthroplasty: a randomized clinical trial. J Knee Surg 32:171–179
Jules-Elysee KM, Lipnitsky JY, Patel N et al (2011) Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med 36:36–40
Abdel MP, Chalmers BP, Taunton MJ et al (2018) Intravenous versus topical tranexamic acid in total knee arthroplasty: both effective in a randomized clinical trial of 640 patients. J Bone Joint Surg Am 100:1023–1029
Fillingham YA, Ramkumar DB, Jevsevar DS et al (2019) Tranexamic acid in total joint arthroplasty: the endorsed clinical practice guides of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. Reg Anesth Pain Med. https://doi.org/10.1136/rapm-2018-000024
Elias KM, Stone AB, McGinigle K et al (2019) The reporting on ERAS compliance, outcomes, and elements research (RECOvER) checklist: a joint statement by the ERAS and ERAS USA societies. World J Surg 43:1–8
Risberg B (1985) The response of the fibrinolytic system in trauma. Acta Chir Scand Suppl 522:245–271
Benoni G, Lethagen S, Fredin H (1997) The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res 85:195–206
Hiippala ST, Strid LJ, Wennerstrand MI et al (1997) Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 84:839–844
Yang Z-G, Chen W-P, Wu L-D (2012) Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 94:1153–1159
Maniar RN, Kumar G, Singhi T et al (2012) Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res 470:2605–2612
Molloy DO, Archbold HAP, Ogonda L et al (2007) Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br 89:306–309
Alshryda S, Sarda P, Sukeik M et al (2011) Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br 93:1577–1585
Xie J, Ma J, Yao H et al (2016) Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplasty 31:2458–2464
Li D, Wang Q, Zhao X et al (2021) Comparison of intravenous and topical dexamethasone for total knee arthroplasty: a randomized double-blinded controlled study of effects on dexamethasone administration route and enhanced recovery. J Arthroplasty 36:1599–1606
Xie J, Hu Q, Huang Q et al (2017) Comparison of intravenous versus topical tranexamic acid in primary total hip and knee arthroplasty: an updated meta-analysis. Thromb Res 153:28–36
Lee D-H, Choi J, Nha K-W et al (2011) No difference in early functional outcomes for mini-midvastus and limited medial parapatellar approaches in navigation-assisted total knee arthroplasty: a prospective randomized clinical trial. Knee Surg Sports Traumatol Arthrosc 19:66–73
Lei Y, Xie J, Huang Q et al (2020) Is there a maximal effect of tranexamic acid in patients undergoing total knee arthroplasty? A randomized controlled trial. MedComm 2020(1):219–227
Xu H, Xie J, Yang J et al (2021) Synergistic effect of a prolonged combination course of tranexamic acid and dexamethasone involving high initial doses in total knee arthroplasty: a randomized controlled trial. J Knee Surg. https://doi.org/10.1055/s-0041-1739197
Cui D, Lei Y, Xu H et al (2019) Efficacy and safety of a loading high-dose tranexamic acid followed by postoperative five doses in total hip arthroplasty: a randomized controlled trial. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 33:935–939
Cao G, Chen G, Huang Q et al (2019) The efficacy and safety of tranexamic acid for reducing blood loss following simultaneous bilateral total knee arthroplasty: a multicenter retrospective study. BMC Musculoskelet Disord 20:325
Lei Y, Xie J, Huang Q et al (2020) Additional benefits of multiple-dose tranexamic acid to anti-fibrinolysis and anti-inflammation in total knee arthroplasty: a randomized controlled trial. Arch Orthop Trauma Surg 140:1087–1095
Zhang S, Xie J, Cao G et al (2021) Six-dose intravenous tranexamic acid regimen further inhibits postoperative fibrinolysis and reduces hidden blood loss following total knee arthroplasty. J Knee Surg 34:224–232
National Health Commission of the People's Republic of China (2001) Technical specifications of clinical blood transfusion. National Health Commission of the People's Republic of China, Beijing. http://www.nhc.gov.cn/wjw/gfxwj/200111/2c93606209ec4a25ad9241787f9f7404.shtml.
Gross JB (1983) Estimating allowable blood loss: corrected for dilution. Anesthesiology 58:277–280
Nadler SB, Hidalgo JH, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224–232
Gal TJ, Cooperman LH (1975) Hypertension in the immediate postoperative period. Br J Anaesth 47:70–74
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Cao G, Xie J, Huang Z et al (2018) Efficacy and safety of multiple boluses of oral versus intravenous tranexamic acid at reducing blood loss after primary total knee arthroplasty without a tourniquet: a prospective randomized clinical trial. Thromb Res 171:68–73
Dennis DA, Kittelson AJ, Yang CC et al (2016) Does tourniquet use in tka affect recovery of lower extremity strength and function? A randomized trial. Clin Orthop Relat Res 474:69–77
Herndon CL, Grosso MJ, Sarpong NO et al (2020) Tibial cement mantle thickness is not affected by tourniquetless total knee arthroplasty when performed with tranexamic acid. Knee Surg Sports Traumatol Arthrosc 28:1526–1531
Goel R, Rondon AJ, Sydnor K et al (2019) Tourniquet use does not affect functional outcomes or pain after total knee arthroplasty: a prospective, double-blinded, randomized controlled trial. J Bone Joint Surg Am 101:1821–1828
Alcelik I, Pollock RD, Sukeik M et al (2012) A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty 27:331–340
Tai T-W, Chang C-W, Lai K-A et al (2012) Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 94:2209–2215
Sehat KR, Evans RL, Newman JH (2004) Hidden blood loss following hip and knee arthroplasty correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 86:561–565
Kiss H, Raffl M, Neumann D et al (2005) Epinephrine-augmented hypotensive epidural anesthesia replaces tourniquet use in total knee replacement. Clin Orthop Relat Res. https://doi.org/10.1097/01.blo.0000161825.90633.12
Lieberman JR, Huo MM, Hanway J et al (1994) The prevalence of deep venous thrombosis after total hip arthroplasty with hypotensive epidural anesthesia. J Bone Joint Surg Am 76:341–348
Juelsgaard P, Larsen UT, Sørensen JV et al (2001) Hypotensive epidural anesthesia in total knee replacement without tourniquet: reduced blood loss and transfusion. Reg Anesth Pain Med 26:105–110
Wesselink EM, Kappen TH, Torn HM et al (2018) Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth 121:706–721
Walsh M, Devereaux PJ, Garg AX et al (2013) Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 119:507–515
Zhou K, Ling T, Wang H et al (2017) Influence of tourniquet use in primary total knee arthroplasty with drainage: a prospective randomised controlled trial. J Orthop Surg Res 12:172
Leurcharusmee P, Sawaddiruk P, Punjasawadwong Y et al (2018) The possible pathophysiological outcomes and mechanisms of tourniquet-induced ischemia-reperfusion injury during total knee arthroplasty. Oxid Med Cell Longev 2018:8087598
Acknowledgements
We gratefully acknowledge the surgeons and nurses from the Department of Orthopedics of West China Hospital.
Funding
This study was supported by the National Health Commission of the People’s Republic of China (project no. ZX2021007). Funds were used to cover transportation for patient follow-up.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Hong Xu, Xing Wang, Menghan Liu, and Jinwei Xie. The first draft of the manuscript was written by Wenyu Jiang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Biomedical Ethics Committee of Sichuan University West China Hospital (date February 8, 2022/no. 2021–1699). Each patient provided written informed consent.
Consent for publication
Not applicable.
Competing interests
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, W., Wang, X., Xu, H. et al. Tourniquets can further reduce perioperative blood loss in patients on dexamethasone and tranexamic acid during cemented total knee arthritis: a single-center, double-blind, randomized controlled trial. J Orthop Traumatol 24, 17 (2023). https://doi.org/10.1186/s10195-023-00698-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-023-00698-3