Abstract
Electrical stimulation is used to elicit muscle contraction and can be utilized for neurorehabilitation following spinal cord injury when paired with voluntary motor training. This technology is now an important therapeutic intervention that results in improvement in motor function in patients with spinal cord injuries. The purpose of this review is to summarize the various forms of electrical stimulation technology that exist and their applications. Furthermore, this paper addresses the potential future of the technology.
Similar content being viewed by others
Epidemiology
Globally, approximately 250,000 to 500,000 new spinal cord injury (SCI) cases occur every year [1]. Blunt force trauma is primarily responsible for SCI, with motor vehicle crashes serving as the leading cause of injury (38.2%), followed by falls (32.3%) [2]. Medical expenses over US $3 billion are spent annually on managing SCI, and individual costs can range from US $380,000 to US $1,160,000 in the first year alone, and between US $46,000 and US $202,000 for each subsequent year [2].
Sequelae
Neurologic injury of the spinal cord affects nearly every physiologic system, and patients can present with a multitude of symptoms that drastically influence their function and quality of life. The SCI level determines which systems are affected and has a significant impact on the potential rehabilitation and final functional status of the patient.
Musculoskeletal system
While C1–C4 SCI typically results in tetraplegia, lower cervical (C5–C8) SCI can spare varying degrees of upper extremity function. The C5 nerve root primarily innervates the deltoid muscle to perform shoulder abduction, but is also responsible elbow flexion. Accordingly, C5 complete SCI (ASIA A) results in complete dependence for transfers and assistance for activities of daily living. The C6 nerve root controls wrist extension and biceps flexion, the C7 nerve root controls elbow extension and wrist flexion, and the C8 nerve roots controls finger flexion. SCI below C6 results in relatively greater independence, with patients able to achieve transfers either with the assistance of a transfer board (C6) or independently (C7/C8). These patients require less assistance and fewer adaptive aids for activities of daily living. Any complete level thoracic SCI results in paraplegia, however, SCI distal to L2 level may spare varying lower extremity function.
Damage to descending spinal cord tracks results in hyperexcitability and spasticity [3, 4]. Spasticity is a velocity-dependent increase in muscle tone due to a hyperexcitable stretch reflex [5]. Spasticity may potentially have beneficial effects by promoting venous return, decreasing the incidence of orthostatic hypertension and deep venous thrombosis, increasing stability, and facilitating activities such as transfers [3, 6, 7]. However, these must be weighed against the negative effects that include contractures, gait disturbances, decreased mobility, and pain [3, 8, 9].
Due to mobility limitations, paraplegia or tetraplegia patients do not load their spine or limbs, disturbing bone homeostasis as a result of mechanical unloading. Cessation of weight bearing in these patients leads to increased bone resorption and suppressed bone formation. The resulting osteoporosis is typically isolated to the long bones below the level of injury, increasing the risk of fragility fractures [10,11,12,13].
Integumentary system
One of the most common adverse events following an SCI is pressure ulcers due to insensate regions. Ulceration occurs due to persistent pressure over bony prominences as a result of immobility, poor nutrition, and changes in skin physiology including deficient vascular reactions to catecholamine signaling and decreased fibroblast activity. These changes delay the natural wound healing capabilities below the level of the injury, resulting in ulceration [14, 15]. The annual incidence of pressure ulcers in SCI patients ranges from 20% to 31%, with the resulting increased healthcare utilization approximately quadrupling annual costs compared with SCI patient without ulcers [16, 17].
Cardiopulmonary system
SCI in the cervical or high thoracic regions can disrupt respiratory muscle function, ranging from exercise intolerance to complete respiratory failure requiring mechanical ventilation assistance [18]. In patients with prolonged ventilation, tracheostomy may be required. Poor respiratory muscle recruitment in combination with inhibited reflexes results in impaired cough, bronchospasm, and increased secretions, predisposing SCI patients to pneumonia, atelectasis, and exacerbation of respiratory failure [14, 19].
SCI patients also have increased risk of ischemic heart disease because of the increased prevalence of coronary artery disease (CAD) and hypertension after SCI [20]. The prevalence of symptomatic cardiovascular disease ranges from 30% to 50% compared with 5–10% in matched able-bodied populations [21, 22]. CAD risk factors, including hyperlipidemia, diabetes, and obesity, that exist within the SCI population have primarily been attributed to the sedentary nature of SCI patients [23,24,25,26,27].
Sympathetic nervous system
SCI proximal to T6 level may result in autonomic dysreflexia, affecting autonomic responses to demands on vascular tone and heart rate, with greater severity of dysregulation associated with higher levels of injury [28, 29]. Autonomic dysreflexia results in sympathetic over activity causing hypertension that increases the risk of stroke, pain, and hemodynamic instability. Parasympathetic compensation, including bradycardia and vasodilation, occurs only above the level of the injury, resulting in sweating, chills, headache, and flushing [30, 31]. Dysreflexia is often initiated by noxious stimuli below the level of the spinal cord injury, including cutaneous or visceral etiologies, but is most often triggered by a urologic source such as urinary tract infection or bladder distention [32]. Injuries below T6 do not typically result in autonomic dysreflexia due to the intact splanchnic innervation [33].
Urinary system
SCI can disrupt both storage and emptying of the bladder. The majority of bladder dysfunction results from detrusor overactivity causing urge incontinence. Patients can also have detrusor sphincter dyssynergia, where the bladder contracts against a hyperactive closed sphincter leading to vesicoureteral reflux [34,35,36,37,38]. Detrusor areflexia has also been noted in SCI patients with involvement of lower motor neurons resulting in chronic urinary retention with incomplete emptying and overflow incompetence [38]. Due to these conditions, many patients require intermittent catheterization or indwelling catheters that increase the risk of developing urinary tract infections (UTIs) [38,39,40,41]. Patients with SCI are also noted to have an increased incidence of nephrolithiasis secondary to immobilization hypercalciuria, which may also predispose patients to UTIs [14, 38, 42,43,44,45].
Reproductive system
In addition to urologic impairments, SCI often results in sexual dysfunction. The incidence of impotence in men after SCI is approximately 75%, where the level of the injury dictates the type of sexual dysfunction. If there is a lower motor neuron lesion at the level of the sacral roots, parasympathetic innervation will be interrupted and reflexogenic erections are impacted (i.e., tactile stimulation resulting in an erection). Alternatively, psychogenic erections are mediated through sympathetic pathways originating from T10–T12. As such, psychologically mediated erections are possible in patients with injuries caudal to T12 [38]. There is a paucity of literature on sexual dysfunction in women with SCI, but an impaired ability to achieve orgasm after SCI has been described [38].
Classifications of neurologic injury
The Frankel scale was introduced in 1969 as a 5-point scale to grade SCI [46]. Patients are classified as complete (grade A), sensory only (grade B), motor useless (grade C), motor useful (grade D), or no neurological deficit/complete recovery (grade E). Continued use of this scale was limited by its subjective nature in judging “usefulness” of any remaining motor movements and its failure to account for the level of injury [46]. The American Spinal Injury Association published the International Standards for Neurological Classification of Spinal Injury in 1982 [47]. This classification has evolved into the current American Spinal Injury Association Impairment Scale (AIS) [48]. In contrast to the Frankel system, the AIS improves reproducibility via standardized testing of myotomes and dermatomes to identify the level of injury [49, 50]. Additionally, the AIS differentiates between complete and incomplete injuries.
The AIS is now the international standard for evaluation and classification of patients with SCI [50]. The scale grades A–E: Patients with Grade A have complete spinal cord injuries and as such, have no motor or sensory function (including sacral roots) distal to the level of injury. Patients with Grade B have some sensory function, but no motor function below the level of injury. Grade C injuries consist of a motor strength less than 3/5 in more than half of the major muscle groups below the level of injury, while Grade D injuries have a motor grade 3/5 or greater. Patients with Grade E have full motor and sensory function after sustaining a SCI [48].
Electrical simulation
Spinal cord injury is a discontinuity syndrome that disrupts efferent and afferent pathways, including the descending motor fibers from the motor cortex to the spinal motor neurons and the ascending somatosensory fibers from the peripheral nervous system through the spinal cord and to the brain [51]. In other regions of the body, conduction blockage is resolved with regrowth, regeneration, and sometimes functional reconnectivity of axons to the end organ resulting in functional recovery. However, the central nervous system has impaired ability to restore neural circuits across a lesion. Accordingly, scientific innovations have been created to bypass the area of injury and reconnect end organ function.
The use of electrical stimulation after spinal cord injuries has been utilized for over half a century [52]. One of the earliest uses of electrical stimulation in the 1960s utilized electrical current to stimulate the peroneal nerve to initiate muscle function and correct foot drop in the setting of stroke-related hemiplegia [53]. Since then, various types of electrical stimulation have been developed and employed to conserve the function of the aforementioned physiologic systems (Table 1).
Complete SCI prevents any signal from descending below the level of the injury due to incomplete circuitry. Even in completely injured patients, some circuits are spared, although these circuits are often not sufficient to establish an adequate level of excitability to stimulate motor neurons caudal to the injury. Electrical stimulation is believed to work by inducing neuroplastic changes at synapses within the spinal cord. Neuroplasticity is the process in which axons and synapses reorganize and adapt to their cellular environment.
After SCI, axon growth can include collateral sprouting of spared and injured axons, synaptic remodeling, and axon regeneration, albeit to a lesser extent than that which occurs outside the central nervous system [54]. Axonal sprouting and synaptic remodeling result in circuit reorganization, while axonal regeneration involves the regrowth of transected axons. Electrical stimulation induces neuroplasticity by increasing the baseline level of spinal excitability such that low levels of input result in voluntary motor function [55]. It has been hypothesized that the combination of electrical stimulation with voluntary motor commands is necessary to induce neuroplastic changes. When descending signals from the brain reach the corticospinal anterior horn synapse at the same time as antidromic signals traveling up the peripherally stimulated nerve by electrical stimulation, the synapse is strengthened and increases the probability of subsequent firing in a Hebbian-type learning effect, which postulates that an increase in synaptic efficiency arises from repeated stimulation [56]. This synaptic plasticity likely involves descending motor axons, proprioceptive afferents, motor neurons, and interneurons. By using electrical stimulation paired with voluntary motor training, the elicited neuroplasticity results in improvements in motor function.
Transcutaneous electrical neural stimulation
Transcutaneous electrical neural stimulation (TENS) is a surface applied neuromodulation system that has been utilized in the treatment of various types of chronic pain, including noninvasive neuropathic pain relief through stimulation of sensory A-beta fibers and blocking of pain signals transmitted via A-delta and C-nociceptive fibers [57,58,59]. TENS is also used in the management of spasticity through a mechanism of neuroplasticity or modulation of inhibitory circuits [60,61,62]. TENS has been shown to enhance vibratory inhibition of the H reflex, the electrical equivalent of the monosynaptic stretch reflex, which has been attributed to presynaptic inhibition [60]. TENS treatment for spasticity enhances presynaptic inhibition, which is intrinsically suppressed in SCI patients. Furthermore, TENS resembling sensory feedback has been shown to induce short-term neuroplasticity by increasing the strength of reciprocal Ia inhibition between ankle flexor and extensor muscles [61].
Neuromuscular electrical stimulation
Neuromuscular electrical stimulation (NMES) is electricity applied across the surface of the skin, and involves direct stimulation of targeted nerves to contract paralyzed muscles and increase muscle strength. NMES is thought to improve spasticity via disynaptic reciprocal inhibition in which the activation of one muscle produces an inhibition of the opposing muscle group [63, 64]. NMES is used to reverse muscle mass loss and improve functional movement similar to traditional muscle exercise [65, 66]. Furthermore, NMES is used in conjunction with repetitive movement therapy to facilitate motor relearning [67]. For example, NMES combined with standard rehabilitation has been shown to increase recovery of wrist extension over standard care in hemiplegic patients [68]. These therapeutic applications may lead to an effect that enhances but does not directly provide function. When NMES is used to directly accomplish functional tasks, it is called functional electrical stimulation (FES).
Functional electrical stimulation
Functional electrical stimulation (FES) is a subtype of NMES that involves applying electrical stimuli to paralyzed nerves or muscles to induce muscular contraction in order to complete a functional task [69]. Conventional FES has been used in neurorehabilitation for tasks such as rowing or cycling [70, 71]. FES in neurorehabilitation is thought to support the rewiring and regeneration of damaged synaptic connections [72].
Stimuli
FES uses surface or implantable electrodes to deliver electrical stimuli. The placement of the electrode determines the selection of muscles stimulated and resulting movements. However, the optimal location of electrode placement and intensity of electrical stimuli requires trial and error to isolate the desired movement. The intensity of the electrical stimuli is determined by adjusting the duration and amplitude of the pulse (Fig. 1). Pulse duration is the time in which the stimulation is present, while pulse amplitude is the magnitude of the stimulation and determines which nerve fibers respond to the stimulation. As the intensity of the pulse increases, in either amplitude or duration, the current spreads and activates a larger cross-sectional area of skeletal muscle increasing the force exerted. Large nerves, which innervate large motor units, have the lowest threshold for stimulation and are recruited first, followed by small neurons and motor units. This phenomena is known as reverse recruitment and is the opposite of the physiologic size principle of motor neuron activation [73]. Unfortunately, this early recruitment of large muscles commonly leads to muscle fatigue, which may be mitigated to some degree through the modification of pulse frequency. Pulse frequency is the rate at which stimulation pulses are delivered. By increasing the pulse frequency, individual muscle twitches compound into a sustained contraction to produce movement called tetanic contraction. A minimum frequency of 16–20 Hz is required to induce contractions [74]. Higher frequencies create stronger contractions but also exacerbate muscle fatigue. Thus, a range of 20–50 Hz are typically used in FES.
Pulses used in electrostimulation can be either monophasic, also known as direct current, or biphasic, also known as alternating current (Fig. 2). Monophasic pulses consist of a unidirectional pulse, whereas biphasic pulses are bidirectional with a positive and negative phase. The primary risk of monophasic pulses is thermal injury to surrounding tissue. Biphasic pulses can alternate anode and cathode electrodes (alternating biphasic pulses), which is believed to be safer for surrounding tissue. Biphasic pulses have a net charge of zero as the initial phase elicits an action potential in nearby nerves and the second phase balances the charge injection to protect surrounding tissue.
FES is either controlled as an open or closed loop system (Fig. 3). Open loop systems apply electrical current using fixed settings and do not incorporate biofeedback, and therefore lack the ability to self-correct. Alternatively, closed loop systems continuously relay contraction force and joint position information via sensors back to a computer to modulate input [75].
Therapeutic use
FES plays a prominent role in rehabilitation following SCI, mainly to restore extremity function. FES has been shown to increase muscle power output and resistance [76]. Multiple studies have validated the use of FES in helping to restore upper extremity function following SCI [77,78,79,80]. FES has also been used in rehabilitation of the lower extremity to improve gait parameters such as foot pulling acceleration, swing power, and ground impact force, ultimately resulting in improved walking speeds and more efficient system of muscle strength for gait [81,82,83,84,85]. A study using ankle weights to measure improvement in muscle strength after FES treatment for AIS A–C patients unable to stand demonstrated an average of 2–4× increase in power output in a 12 week study [76]. Additionally, FES has also been shown to improve patient transitions, spasticity, cardiovascular function, and pain [86,87,88,89]. Lastly, recent evidence has found that electrical stimulation increases the success rate of spinal fusion [90]. As such, functional or direct electrical stimulation could potentially be used to reverse and counter the bone loss and risk of fragility fracture in SCI patients.
Neuroprosthesis
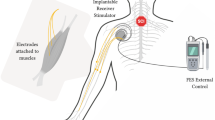
FES technology has been integrated into neuroprostheses to control paralyzed muscles and improve functional independence. These systems comprise four major parts: the electrical stimulator, electrodes, sensors, and an orthosis. The electrical stimulator generates the electrical discharges that produce muscle contractions. These stimulators contain multiple channels, with each channel consisting of a pair (anode and cathode) of electrodes. Multiple channels are used to stimulate multiple muscles individually to produce functional movement. Electrodes are the interface between the external circuitry and the tissue and can be transcutaneous or implantable. Transcutaneous systems are noninvasive, do not require surgery, and are easy to reposition [74]. They can be connected to an external stimulator worn around the body that regulates and initiates the electrical stimulation, however, these systems are not suitable for stimulation of deep muscles and do not consistently achieve effective stimulations [73]. Implanted electrodes are surgically placed in the body, which allows for precise placement and direct stimulation of the desired muscles, resulting in repeatable and well-controlled contractions [73]. While reliable and effective, they have higher risks of complications, such as infections, due to their invasive nature. Additionally, their placement cannot be modified without additional surgery [51, 91, 92]. Sensors provide the biofeedback for the neuroprosthesis to achieve maximum functionality. Feedback-based control systems monitor the musculoskeletal system to alter the stimulation parameters in real time to achieve the desired movement. Finally, an orthosis provides additional structural assistance to perform desired movement by preventing muscle fatigue and helping patients conserve energy [93].
Hand and arm control are the most desired functions for patients with cervical SCI. FES neuroprosthesis have been developed to facilitate upper limb functions of reaching and grasping. The Freehand system developed by Hunter Peckham, Ron Triolo, and colleagues at the Cleveland FES Center was the first hand system to be granted United States Food and Drug Administration (FDA) approval [94]. The system consisted of implantable electrodes and a joystick to control the device. While it is no longer commercially available, a newer version, referred to as the implanted stimulator-telemeter (IST-12), was developed by the same team and has shown promising results in a clinical trial for improving the ability to grasp and manipulate objects [95].
After SCI, atrophied trunk musculature often lacks the required forces to control posture [96, 97]. Continuous FES can be used to stiffen trunk and hip extensors to stabilize the torso, correct kyphotic posture, improve ventilation, and normalize lateral vertebral alignment [98, 99]. Implanted electrodes at L1–L2 recruit lumbar erector muscles in combination with electrodes that activate the gluteus maximus improve trunk and hip extension. By activating these muscles, patients experience improved seated stability, seated posture, and enhanced bimanual reach [98]. However, this improvement cannot be maintained without constant stimulation. Further research is underway in a clinical trial investigating a trunk neuroprosthesis that is surgically implanted to facilitate trunk stability while sitting [100].
Neuroprostheses have shown promise in restoring the ability to stand and walk. FES used in combination with an ankle foot orthosis to provide support has helped patients activate the muscles in the lower extremity necessary for standing and walking [93]. Patients with implanted neuroprosthesis electrodes that continuously activate the erector spinae and gluteus maximus muscles for trunk and hip extension have been shown to maintain standing for greater than 10 minutes [101]. This small time frame enables patients to access wheelchair inaccessible areas and to utilize their upper extremities for activities other than maintaining balance with assistive devices. Furthermore, neuroprostheses have successfully reduced the torque and power output needed to initiate walking movement [102]. One of the most successful neuroprostheses for walking is Parastep. Parastep is an FDA approved device that uses transcutaneous electrodes over the peroneal nerves to allow ambulation [103]. Lower extremity neuroprostheses still face significant limitations due to the rapid onset of muscle fatigue and upper-body effort required to maintain balance with an ambulatory assistive device. A clinical trial is currently underway to investigate a new standing neuroprosthesis that uses multiple contact peripheral electrodes to slow fatigue onset and increase standing duration [104].
Phrenic nerve pacing
High cervical spinal injuries carry the risk of altering respiratory function, which can result in respiratory failure. An alternative to ventilator dependence is diaphragmatic pacing via electrical stimulation of the phrenic nerve [105]. Phrenic nerve pacing has been used successfully for over 30 years, and a variety of implanted systems have been developed and commercialized [106]. Phrenic pacing has been shown to reduce time on the ventilator and may provide a full-time alternative to a ventilator [107]. Phrenic nerve pacing requires intact nerve function. However, pacing was recently achieved in patients with high cervical SCI (C3–5) and loss of phrenic nerve function via intercostal nerve grafting and implantable electrodes [108].
Spinal cord stimulation
Spinal cord stimulation (SCS) is a neuromodulation technique used to treat neuropathic pain by virtue of its purported effect of blocking the transmission of pain signals through nociceptive nerve fibers entering the dorsal horn, similar to TENS [109]. SCS involves transcutaneous electrodes placed on the skin over the vertebral column or implanted epidural electrodes in the dorsal spinal cord. Increasing evidence shows that SCS also improves motor function via neuroplasticity following SCI [60, 110]. Recently, transcutaneous SCS has been shown to increase upper and lower extremity function, comparable to implanted SCS [111,112,113,114,115,116,117]. These findings are controversial and the rationale for the current Up-LIFT clinical trial that seeks to evaluate the effectiveness of noninvasive electrical SCS in treating upper extremity functional deficits in patients with chronic tetraplegia [118]. The advantages of transcutaneous SCS include its noninvasive application, affordability, and compatibility with conventional and commercially available stimulation devices. Recent advances in SCS include the delivery of spatiotemporal stimulation based upon gait kinematics and locomotor performance. However, this technology will require implantation for the foreseeable future to target specific areas of the spinal cord and stimulate unique muscles with precise timing. Implanted devices that can apply complex spatiotemporal patterns have reproduced voluntary control of locomotion in severely paralyzed patients [119,120,121]. Clinical trials are underway evaluating SCS in recovering lower extremity, bladder, bowel, and sexual function [122, 123]. Additionally, both transcutaneous and implanted SCS have been shown to improve autonomic cardiovascular dysfunction that occurs after SCI [124].
Autonomic neuromodulation
SCI disrupts sympathetic vasomotor control, resulting in severe cardiovascular dysfunction. While pharmacological treatment of autonomic nervous system (ANS) regulation has demonstrated limited effectiveness, device-based neuromodulation has been shown to successfully modulate the cardiac ANS [125, 126]. Vagal nerve stimulation (VNS) has been shown to promote synaptic plasticity and improve autonomic instability and motor control in preclinical models with the potential to treat dysautonomia related to SCI [127,128,129,130].
An alternative method of autonomic neuromodulation is translingual neurostimulation. Translingual neurostimulation is a noninvasive method used to elicit neural changes in cranial nerves by targeting the anterior portion of the tongue activating the lingual branch of the trigeminal nerve [131, 132]. Prior research has shown that this method induces changes in the brainstem and cerebellum, specifically targeting areas important for postural drive [132,133,134,135]. As such, studies have shown improvement in balance and gait function in patients following SCI, stroke, and traumatic brain injury (TBI) [136,137,138].
Sacral nerve stimulation
Sacral nerve stimulation (SNS) has been established as a treatment for urinary retention, frequency, and incontinence [139, 140]. Sacral nerve stimulation restores normal bladder function by facilitating storage and voiding and suppressing reflex bladder activity through adaptive neuronal plasticity [141]. SNS has successfully treated neurogenic bladder dysfunction via implanted sacral and transcutaneous sacral root, posterior tibial, and dorsal genital nerve stimulation [142,143,144,145].
Galvanic vestibular stimulation
Galvanic vestibular stimulation (GVS) applies current at the mastoid process and activates afferent fibers of the vestibular nerve. Vestibulospinal neurons converge on spinal interneurons, promoting inhibitory or excitatory actions. These actions affect the tone of postural muscles, where stimulation of the anode results in hypotonia and stimulation of the cathode results in hypertonia. GVS can modulate the vestibulospinal tract and has been used to supplement the neurological examination by diagnosing and quantifying vestibulospinal deficits in patients with incomplete SCI [146]. GVS has been shown to reduce spasticity in SCI patients, and increase postural stability [147, 148].
Transcranial direct current stimulation
Transcranial direct current stimulation (tDCS) is a noninvasive approach that delivers low-intensity direct current via electrodes placed on the head. tDCS is hypothesized to promote neuronal plasticity by altering membrane potential and cortical excitability [149]. Whether tDCS is depolarizing or hyperpolarizing, and inhibitory or excitatory, depends on the exact spatial locations of the contacts, the current path, the underlying geometry of the brain, and the degree of shunting to the scalp and skull. tDCS has been combined with motor training to promote activity-dependent neuronal plasticity, and has been shown to improve manual dexterity [150,151,152]. However, improvements in lower extremity motor function remain controversial [153]. A clinical trial is currently underway to investigate transcutaneous direct current stimulation (tcDCS) on walking function in individuals with incomplete SCI [154]. Variations of tDCS include alternating current stimulation (ACS) and oscillating field stimulation (OSF). ACS delivers transcranial alternating current electrical stimulation and has been shown to decrease pain perception and increase muscle work in SCI patients [155]. OSF has shown promise for remyelination and axon regeneration in preclinical models, but failed to significantly improve motor status in humans [156,157,158].
Future directions
Current trends in health care delivery have encouraged research in methods to deliver FES on an outpatient basis. One solution is a garment-based stimulation technology developed by the textile computing company Myant (Toronto, Canada) (Fig. 4). These garments combine cloth with silver thread or conductive layers to embed electrodes into the clothing to deliver electrical stimulation. These wearable garments should be customizable, cost effective, versatile, and durable compared with alternative options, and allow for independent application by the patient [159].
Previously, TENS/FES/SCS has been used to restore function using various command interfaces including electromyography (EMG), voice recognition, mouth sticks, chin-controlled joysticks, sip-and-puff, inertial measurement units (accelerometer, gyroscope), eye gaze, head tracking, and tongue movement [160,161,162,163,164,165,166,167]. Brain–computer interfaces (BCI) are an emerging technology with the potential to be used in SCI rehabilitation. Electroencephalogram (EEG)-based BCI has been shown to translate a task-related motor intention neural signal into a specific command [168]. Recently, progress has been made in using intracortical brain–computer interfaces to interpret intended movement signals and command transcutaneous and intramuscularly implanted FES electrodes to generate the intended movements of reaching and grasping [169,170,171,172,173]. Furthermore, clinical trials are underway investigating a spine interface that will bridge spinal cord lesions by interpreting neural information above a lesion and transmitting it to electrodes below the lesion [174].
Neuromodulation for SCI
The direction of care for patients with SCI can often be complex given the numerous modalities available to assist in rehabilitation. Spinal surgeons may be inclined to place any number of implantable devices during the index spinal cord stabilization surgery. Unfortunately, due to the nature of SCI, the full extent of a patient’s functional limitations may not be known until significant time has passed. As such, this poses a challenge for determining the proper intervention early in the disease course when early intervention could drastically improve long-term functional recovery.
Preoperatively, spine surgeons must consider the extent of SCI using objective scales such as the AIS, as well as their own clinical judgement regarding the long-term recovery of the patient. In the future, surgeons may utilize advanced diffusion tactography sequences on magnetic resonance imaging (MRI), which have been shown to detect functional changes in SCI patients to help predict outcomes and guide treatment [175]. Although the entire course of a SCI is difficult to predict at onset, certain extremes of injury can be predicted based on initial examination findings. These dysfunctional injuries may benefit from early intervention in high probability areas of functional impairment. For example, a patient with complete cord transection resulting from high cervical spinal injury will almost surely be placed on a ventilator for immediate life-saving support and, therefore, have a high likelihood of showing early improvement with a phrenic nerve pacer to prevent atrophy of respiratory musculature. The implication of such interventions would be the prevention of multiple surgeries when anticipated sequelae could be prevented during the index procedure.
Early neurorehabilitation with electrical stimulation has the potential to reduce morbidity and mortality in patients with SCI. Future research should focus on ways to organize and plan early management to prevent unnecessary surgeries, while increasing functionality and recovery. Additionally, further consideration is required to compare the risks and benefits of these interventions as technology continues to flourish in the wake of faster, more precise, and effective techniques.
Conclusion
Electrical stimulation can be used in various forms to improve the well-being and functionality of patients with SCI. The scope of electrical stimulation continues to grow as more advanced technologies and interventions are developed and studied. The neuroplasticity induced by electrical stimulation portends a promising future for developing better therapeutic interventions that can lessen the functional disability and enhance the quality of life of patients with SCI. The prevalence of electrical stimulation will likely increase in the future, with neuroprosthetic devices playing an important role in rehabilitation.
Availability of data and materials
Not applicable.
Abbreviations
- SCI:
-
Spinal cord injury
- CAD:
-
Coronary artery disease
- UTI:
-
Urinary tract infection
- TENS:
-
Transcutaneous electrical neural stimulation
- NEMS:
-
Neuromuscular electrical stimulation
- SCS:
-
Spinal cord stimulation
- ANS:
-
Autonomic nervous system
- VNS:
-
Vagal nerve stimulation
- SNS:
-
Sacral nerve stimulation
- GVS:
-
Galvanic vestibular stimulation
- tDCS:
-
Transcranial direct current stimulation
- tcDCS:
-
Transcutaneous direct current stimulation
- ACS:
-
Alternating current stimulation
- OSF:
-
Oscillating field stimulation
- BCI:
-
Brain–computer interfaces
- EEG:
-
Electroencephalogram
References
WHO Spinal cord injury. https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury. Accessed 23 Jul 2021
National Spinal Cord Injury Statistical Center (2021) Facts and figures at a glance
Adams MM, Hicks AL (2005) Spasticity after spinal cord injury. Spinal Cord 43:577–586
Satkunam LE (2003) Rehabilitation medicine: 3 management of adult spasticity. CMAJ 169:1173–1179
Lance JW (1980) The control of muscle tone, reflexes, and movement: robert Wartenbeg lecture. Neurology 30:1303–1313. https://doi.org/10.1212/wnl.30.12.1303
Kita M, Goodkin DE (2000) Drugs used to treat spasticity. Drugs 59:487–495
Kirshblum S (1999) Treatment alternatives for spinal cord injury related spasticity. J Spinal Cord Med 22:199–217. https://doi.org/10.1080/10790268.1999.11719570
Nair KPS, Marsden J (2014) The management of spasticity in adults. BMJ 349:g4737
Rekand T, Hagen EM, Grønning M (2012) Spastisitet etter ryggmargsskade. Tidsskr den Nor Laegeforening 132:970–973. https://doi.org/10.4045/tidsskr.10.0872
Battaglino RA, Lazzari AA, Garshick E, Morse LR (2012) Spinal cord injury-induced osteoporosis: pathogenesis and emerging therapies. Curr Osteoporos Rep 10:278–285. https://doi.org/10.1007/s11914-012-0117-0
Reiter AL, Volk A, Vollmar J et al (2007) Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J 16:771–776. https://doi.org/10.1007/s00586-006-0163-3
Shojaei H, Soroush MR, Modirian E (2006) Spinal cord injury-induced osteoporosis in veterans. J Spinal Disord Tech 19:114–117. https://doi.org/10.1097/01.bsd.0000194689.95674.c1
Lazo MG, Shirazi P, Sam M et al (2001) Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 39:208–214. https://doi.org/10.1038/sj.sc.3101139
McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ (1999) Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 80:1402–1410. https://doi.org/10.1016/S0003-9993(99)90251-4
Park JW, Seo CH, Han SH, Lee YG (2011) Sympathetic influence on biomechanical skin properties after spinal cord injury. Spinal Cord 49:236–243. https://doi.org/10.1038/sc.2010.95
Regan MA, Teasell RW, Wolfe DL et al (2009) A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 90:213–231
Stroupe KT, Manheim L, Evans CT et al (2011) Cost of treating pressure ulcers for veterans with spinal cord injury. Top Spinal Cord Inj Rehabil 16:62–73. https://doi.org/10.1310/sci1604-62
Brown R, DiMarco AF, Hoit JD, Garshick E (2006) Respiratory dysfunction and management in spinal cord injury. Respir Care 51:853
Arora S, Flower O, Murray Nicholas PS, Lee BB (2012) Respiratory care of patients with cervical spinal cord injury: a review. Crit Care Resusc 14:64–73
Myers J, Lee M, Kiratli J (2007) Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 86:142–152
Bauman WA, Kahn NN, Grimm DR, Spungen AM (1999) Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord 37:601–616
Levi R, Hultling C, Seiger A (1995) The stockholm spinal cord injury study: 2. associations between clinical patient characteristics and post-acute medical problems. Paraplegia 33:585–594. https://doi.org/10.1038/sc.1995.125
Phillips WT, Kiratli BJ, Sarkarati M et al (1998) Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol 23:641–716
Lee MY, Myers J, Hayes A et al (2005) C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med 28:20–25. https://doi.org/10.1080/10790268.2005.11753794
Yekutiel M, Brooks ME, Ohry A et al (1989) The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia 27:58–62. https://doi.org/10.1038/sc.1989.9
Demirel Ş, Demirel G, Tükek T et al (2001) Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord 39:134–138. https://doi.org/10.1038/sj.sc.3101135
Bauman WA, Adkins RH, Spungen AM, Waters RL (1999) The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord 37:765–771. https://doi.org/10.1038/sj.sc.3100893
West CR, Mills P, Krassioukov AV (2012) Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord 50:484–492
Groah SL, Weitzenkamp D, Sett P et al (2001) The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 39:310–317. https://doi.org/10.1038/sj.sc.3101162
Wu JC, Chen YC, Liu L et al (2012) Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology 78:1051–1057. https://doi.org/10.1212/WNL.0b013e31824e8eaa
Cragg J, Krassioukov A (2012) Autonomic dysreflexia. CMAJ 184:66. https://doi.org/10.1503/cmaj.110859
Krassioukov A, Warburton DE, Teasell R, Eng JJ (2009) A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 90:682–695
Bycroft J, Shergill IS, Choong EAL et al (2005) Autonomic dysreflexia: a medical emergency. Postgrad Med J 81:232–235
Yalla SV, Blunt KJ, Fam BA et al (1977) Detrusor-urethral sphincter dyssynergia. J Urol 118:1026–1029. https://doi.org/10.1016/S0022-5347(17)58286-5
McGuire EJ, Brady S (1979) Detrusor-sphincter dyssynergia. J Urol 121:774–777. https://doi.org/10.1016/S0022-5347(17)56987-6
McGuire EJ, Woodside JR, Borden TA, Weiss RM (1981) Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 126:205–209. https://doi.org/10.1016/S0022-5347(17)54449-3
Perkash I (1993) Long-term urologic management of the patient with spinal cord injury. Urol Clin North Am 20:423–434
Burns AS, Rivas DA, Ditunno JF (2001) The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine (Phila. Pa.1976) 26:S129
Siroky MB (2002) Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 113:67–79. https://doi.org/10.1016/S0002-9343(02)01061-6
Shekelle PG, Morton SC, Clark KA et al (1999) Systematic review of risk factors for urinary tract infection in adults with spinal cord dysfunction. J Spinal Cord Med 22:258–272. https://doi.org/10.1080/10790268.1999.11719579
De Ruz AE, Leoni EG, Cabrera RH (2000) Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol 164:1285–1289. https://doi.org/10.1016/S0022-5347(05)67157-1
Gao Y, Danforth T, Ginsberg DA (2017) Urologic management and complications in spinal cord injury patients: a 40- to 50-year follow-up study. Urology 104:52–58. https://doi.org/10.1016/j.urology.2017.03.006
DeVivo MJ (1985) The risk of bladder calculi in patients with spinal cord injuries. Arch Intern Med 145:428–430. https://doi.org/10.1001/archinte.145.3.428
Mobley HLT, Warren JW (1987) Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol 25:2216–2217. https://doi.org/10.1128/jcm.25.11.2216-2217.1987
Burr RG (1978) Urinary calculi composition in patients with spinal cord lesions. Arch Phys Med Rehabil 59:84–88
Frankel HL, Hancock DO, Hyslop G et al (1969) The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 7:179–192. https://doi.org/10.1038/sc.1969.30
Standards for neurological classification of spinal injury patients (1982) American Spinal Injury Association, Chicago
Kirshblum S, Waring W (2014) Updates for the international standards for neurological classification of spinal cord injury. Phys Med Rehabil Clin N Am 25:505–517
Schuld C, Franz S, Brüggemann K et al (2016) International standards for neurological classification of spinal cord injury: impact of the revised worksheet (revision 02/13) on classification performance. J Spinal Cord Med 39:504–512. https://doi.org/10.1080/10790268.2016.1180831
van Middendorp JJ, Goss B, Urquhart S et al (2011) Diagnosis and prognosis of traumatic spinal cord injury. Glob Spine J 1:001–007. https://doi.org/10.1055/s-0031-1296049
Hamid S, Hayek R (2008) Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: an overview. Eur Spine J 17:1256–1269. https://doi.org/10.1007/s00586-008-0729-3
Thrasher TA, Flett HM, Popovic MR (2006) Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord. https://doi.org/10.1038/sj.sc.3101864
Liberson WT, Holmquest HJ, Scot D, Dow M (1961) Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil 42:101–105
Sofroniew MV (2018) Dissecting spinal cord regeneration perspective. Nature 557:343–350
Grahn PJ, Lavrov IA, Sayenko DG et al (2017) Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc 92:544–554. https://doi.org/10.1016/j.mayocp.2017.02.014
Rushton DN (2003) Functional electrical stimulation and rehabilitation—an hypothesis. Med Eng Phys 25:75–78. https://doi.org/10.1016/S1350-4533(02)00040-1
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150:971–979. https://doi.org/10.1126/science.150.3699.971
Pd WALL (1978) The gate control theory of pain mechanisms. Brain 101:1–18. https://doi.org/10.1093/brain/101.1.1
Pert CB, Snyder SH (1973) Opiate receptor: demonstration in nervous tissue. Science 179:1011–1014. https://doi.org/10.1126/science.179.4077.1011
Levin MF, Hui-Chan CWY (1992) Relief of hemiparetic spasticity by TENS is associated with improvement in reflex and voluntary motor functions. Electroencephalogr Clin Neurophysiol Evoked Potentials 85:131–142. https://doi.org/10.1016/0168-5597(92)90079-Q
Perez MA, Field-Fote EC, Floeter MK (2003) Patterned sensory stimulation induces plasticity in reciprocal Ia inhibition in humans. J Neurosci 23:2014–2018. https://doi.org/10.1523/jneurosci.23-06-02014.2003
Bajd T, Gregoric M, Vodovnik L, Benko H (1985) Electrical stimulation in treating spasticity resulting from spinal cord injury. Arch Phys Med Rehabil. https://doi.org/10.5555/uri:pii:000399938591247X
Crone C (1993) Reciprocal inhibition in man. Dan Med Bull 40:571–581
Alfieri V (1982) Electrical treatment of spasticity. Reflex tonic activity in hemiplegic patients and selected specific electrostimulation. Scand J Rehabil Med 14:177–182
Delitto A, Snyder-Mackler L (1990) Two theories of muscle strength augmentation using percutaneous electrical stimulation. Phys Ther 70:158–164. https://doi.org/10.1093/ptj/70.3.158
Bickel CS, Yarar-Fisher C, Mahoney ET, McCully KK (2015) Neuromuscular electrical stimulation-induced resistance training after SCI: a review of the Dudley protocol. Top Spinal Cord Inj Rehabil 21:294–302
Sheffler LR, Chae J (2007) Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 35:562–590
Powell J, Pandyan AD, Granat M et al (1999) Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke 30:1384–1389. https://doi.org/10.1161/01.STR.30.7.1384
Marquez-Chin C, Popovic MR (2020) Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: a review. Biomed Eng Online 19:34
Martin R, Sadowsky C, Obst K et al (2012) Functional electrical stimulation in spinal cord injury: from theory to practice. Top Spinal Cord Inj Rehabil 18:28–33. https://doi.org/10.1310/sci1801-28
Ho CH, Triolo RJ, Elias AL et al (2014) Functional electrical stimulation and spinal cord injury. Phys Med Rehabil Clin N Am 25:631–654
Beaumont E, Guevara E, Dubeau S et al (2014) Functional electrical stimulation post-spinal cord injury improves locomotion and increases afferent input into the central nervous system in rats. J Spinal Cord Med 37:93–100. https://doi.org/10.1179/2045772313Y.0000000117
Peckham PH, Knutson JS (2005) Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng 7:327–360. https://doi.org/10.1146/annurev.bioeng.6.040803.140103
Popovic MR, Keller T, Pappas IPI et al (2001) Surface-stimulation technology for grasping and walking neuroprostheses: improving quality of life in stroke/spinal cord injury subjects with rapid prototyping and portable FES systems. IEEE Eng Med Biol Mag 20:82–93
Gorman PH (2000) An update on functional electrical stimulation after spinal cord injury. Neurorehabil Neural Repair 14:251–263. https://doi.org/10.1177/154596830001400402
Gorgey AS, Khalil RE, Lester RM et al (2018) Paradigms of lower extremity electrical stimulation training after spinal cord injury. J Vis Exp. https://doi.org/10.3791/57000
Mangold S, Keller T, Curt A, Dietz V (2005) Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord 43:1–13. https://doi.org/10.1038/sj.sc.3101644
Kapadia NM, Bagher S, Popovic MR (2014) Influence of different rehabilitation therapy models on patient outcomes: hand function therapy in individuals with incomplete SCI. J Spinal Cord Med 37:734–743. https://doi.org/10.1179/2045772314Y.0000000203
Popovic MR, Kapadia N, Zivanovic V et al (2011) Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair 25:433–442. https://doi.org/10.1177/1545968310392924
Thorsen R, Dalla Costa D, Chiaramonte S et al (2013) A noninvasive neuroprosthesis augments hand grasp force in individuals with cervical spinal cord injury: the functional and therapeutic effects. Sci World J. https://doi.org/10.1155/2013/836959
Street T, Singleton C (2018) A clinically meaningful training effect in walking speed using functional electrical stimulation for motor-incomplete spinal cord injury. J Spinal Cord Med. https://doi.org/10.1080/10790268.2017.1392106
Johnston TE, Betz RR, Smith BT, Mulcahey MJ (2003) Implanted functional electrical stimulation: an alternative for standing and walking in pediatric spinal cord injury. Spinal Cord. https://doi.org/10.1038/sj.sc.3101392
Maležič M, Hesse S (1995) Restoration of gait by functional electrical stimulation in paraplegic patients: a modified programme of treatment. Paraplegia. https://doi.org/10.1038/sc.1995.28
Kapadia N, Masani K, Craven BC et al (2014) A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med 37:511–524. https://doi.org/10.1179/2045772314Y.0000000263
Shendkar CV, Lenka PK, Biswas A et al (2015) Therapeutic effects of functional electrical stimulation on gait, motor recovery, and motor cortex in stroke survivors. Hong Kong Physiother J. https://doi.org/10.1016/j.hkpj.2014.10.003
Koyuncu E, Nakipoǧlu-Yüzer GF, Doǧan A, Özgirgin N (2010) The effectiveness of functional electrical stimulation for the treatment of shoulder subluxation and shoulder pain in hemiplegic patients: a randomized controlled trial. Disabil Rehabil 32:560–566. https://doi.org/10.3109/09638280903183811
Sabut SK, Sikdar C, Kumar R, Mahadevappa M (2011) Functional electrical stimulation of dorsiflexor muscle: effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation 29:393–400. https://doi.org/10.3233/NRE-2011-0717
Sahin N, Ugurlu H, Albayrak I (2012) The efficacy of electrical stimulation in reducing the post-stroke spasticity: a randomized controlled study. Disabil Rehabil 34:151–156. https://doi.org/10.3109/09638288.2011.593679
Wheeler GD, Andrews B, Lederer R et al (2002) Functional electric stimulation-assisted rowing: increasing cardiovascular fitness through functional electric stimulation rowing training in persons with spinal cord injury. Arch Phys Med Rehabil 83:1093–1099. https://doi.org/10.1053/apmr.2002.33656
Akhter S, Qureshi AR, Aleem I et al (2020) Efficacy of electrical stimulation for spinal fusion: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-61266-x
Gater DR, Dolbow D, Tsui B, Gorgey AS (2011) Functional electrical stimulation therapies after spinal cord injury. NeuroRehabilitation 28:231–248
Smith B, Tang Z, Johnson MW et al (1998) An externally powered, multichannel, implantable stimulator-telemeter for control of paralyzed muscle. IEEE Trans Biomed Eng 45:463–475. https://doi.org/10.1109/10.664202
Triolo RJ, Bieri C, Uhlir J, et al. Implanted functional neuromuscular stimulation systems for individuals with cervical spinal cord injuries: clinical case reports
Peckham PH, Keith MW, Kilgore KL et al (2001) Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch Phys Med Rehabil 82:1380–1388. https://doi.org/10.1053/apmr.2001.25910
Implanted myoelectric control for restoration of hand function in spinal cord injury—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00583804. Accessed 17 May 2021
Spungen AM, Adkins RH, Stewart CA et al (2003) Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 95:2398–2407. https://doi.org/10.1152/japplphysiol.00729.2002
Kukke SN, Triolo RJ (2004) The effects of trunk stimulation on bimanual seated workspace. IEEE Trans Neural Syst Rehabil Eng 12:177–185. https://doi.org/10.1109/TNSRE.2004.827222
Triolo RJ, Bailey SN, Miller ME et al (2013) Effects of stimulating hip and trunk muscles on seated stability, posture, and reach after spinal cord injury. Arch Phys Med Rehabil 94:1766–1775. https://doi.org/10.1016/j.apmr.2013.02.023
Wu GA, Lombardo L, Triolo RJ, Bogie KM (2013) The effects of combined trunk and gluteal neuromuscular electrical stimulation on posture and tissue health in spinal cord injury. PM R 5:688–696. https://doi.org/10.1016/j.pmrj.2013.03.025
A neuroprosthesis for seated posture and balance—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01474148. Accessed 17 May 2021
Triolo RJ, Bailey SN, Foglyano KM et al (2018) Long-term performance and user satisfaction with implanted neuroprostheses for upright mobility after paraplegia: 2- to 14-year follow-up. Arch Phys Med Rehabil 99:289–298. https://doi.org/10.1016/j.apmr.2017.08.470
Ha KH, Murray SA, Goldfarb M (2016) An approach for the cooperative control of FES with a powered exoskeleton during level walking for persons with paraplegia. IEEE Trans Neural Syst Rehabil Eng. https://doi.org/10.1109/TNSRE.2015.2421052
Graupe D, Kohn KH (1998) Functional neuromuscular stimulator for short-distance ambulation by certain thoracic-level spinal-cord-injured paraplegics. Surg Neurol 50:202–207. https://doi.org/10.1016/S0090-3019(98)00074-3
A neuroprosthesis for prolonged standing after SCI using multi-contact peripheral nerve electrodes—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01923662. Accessed 17 May 2021
Chervin RD, Guilleminault C (1997) Diaphragm pacing for respiratory insufficiency. J Clin Neurophysiol 14:369–377
Glenn WWL, Phelps ML (1985) Diaphragm pacing by electrical stimulation of the phrenic nerve. Neurosurgery 17:974–984
Posluszny JA, Onders R, Kerwin AJ et al (2014) Multicenter review of diaphragm pacing in spinal cord injury: successful not only in weaning from ventilators but also in bridging to independent respiration. J Trauma Acute Care Surg 76:303–310. https://doi.org/10.1097/TA.0000000000000112
Nandra KS, Harari M, Price TP et al (2017) Successful reinnervation of the diaphragm after intercostal to phrenic nerve neurotization in patients with high spinal cord injury. Ann Plast Surg 79:180–182. https://doi.org/10.1097/SAP.0000000000001105
Gildenberg PL (2006) History of electrical neuromodulation for chronic pain: table 1. Pain Med 7:S7–S13. https://doi.org/10.1111/j.1526-4637.2006.00118.x
Dietz V, Fouad K (2014) Restoration of sensorimotor functions after spinal cord injury. Brain 137:654–667
Inanici F, Brighton LN, Samejima S et al (2021) Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng 29:310–319. https://doi.org/10.1109/TNSRE.2021.3049133
Inanici F, Samejima S, Gad P et al (2018) Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng 26:1272–1278. https://doi.org/10.1109/TNSRE.2018.2834339
Gerasimenko Y, Gorodnichev R, Moshonkina T et al (2015) Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med 58:225–231
Gad P, Lee S, Terrafranca N et al (2018) Non-invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma 35:2145–2158. https://doi.org/10.1089/neu.2017.5461
Gad P, Gerasimenko Y, Zdunowski S et al (2017) Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front Neurosci 11:333. https://doi.org/10.3389/fnins.2017.00333
Phillips AA, Squair JW, Sayenko DG et al (2018) An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma 35:446–451. https://doi.org/10.1089/neu.2017.5082
Megía García A, Serrano-Muñoz D, Taylor J et al (2020) Transcutaneous spinal cord stimulation and motor rehabilitation in spinal cord injury: a systematic review. Neurorehabil Neural Repair 34:3–12
The Up-LIFT study of non-invasive arc therapy for spinal cord injury—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04697472. Accessed 17 May 2021
Capogrosso M, Wagner FB, Gandar J et al (2018) Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat Protoc 13:2031–2061. https://doi.org/10.1038/s41596-018-0030-9
Wagner FB, Mignardot JB, Le Goff-Mignardot CG et al (2018) Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563:65–93. https://doi.org/10.1038/s41586-018-0649-2
Wenger N, Moraud EM, Gandar J et al (2016) Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med 22:138–145. https://doi.org/10.1038/nm.4025
Epidural stimulation after neurologic damage—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03026816. Accessed 17 May 2021
Neuromodulation: bladder bowel and sexual function in SCI—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04604951. Accessed 17 May 2021
Wecht JM, Harel NY, Guest J et al (2020) Cardiovascular autonomic dysfunction in spinal cord injury: epidemiology, diagnosis, and management. Semin Neurol 40:550–559
Sohinki D, Stavrakis S (2020) New approaches for treating atrial fibrillation: focus on autonomic modulation. Trends Cardiovasc Med 30:433–439
Waldron NH, Fudim M, Mathew JP, Piccini JP (2019) Neuromodulation for the treatment of heart rhythm disorders. JACC Basic to Transl Sci 4:546–562
Chen M, Zhou X, Yu L et al (2016) Low-level vagus nerve stimulation attenuates myocardial ischemic reperfusion injury by antioxidative stress and antiapoptosis reactions in canines. J Cardiovasc Electrophysiol 27:224–231. https://doi.org/10.1111/jce.12850
Ganzer PD, Darrow MJ, Meyers EC et al (2018) Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife. https://doi.org/10.7554/eLife.32058
Sachdeva R, Krassioukov AV, Bucksot JE, Hays SA (2020) Acute cardiovascular responses to vagus nerve stimulation after experimental spinal cord injury. J Neurotrauma 37:1149–1155. https://doi.org/10.1089/neu.2019.6828
Darrow MJ, Torres M, Sosa MJ et al (2020) Vagus nerve stimulation paired with rehabilitative training enhances motor recovery after bilateral spinal cord injury to cervical forelimb motor pools. Neurorehabil Neural Repair 34:200–209. https://doi.org/10.1177/1545968319895480
Ptito M, Moesgaard SM, Gjedde A, Kupers R (2005) Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain 128:606–614. https://doi.org/10.1093/brain/awh380
Wildenberg JC, Tyler ME, Danilov YP et al (2010) Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain Imaging Behav 4:199–211. https://doi.org/10.1007/s11682-010-9099-7
Wildenberg JC, Tyler ME, Danilov YP et al (2011) High-resolution fMRI detects neuromodulation of individual brainstem nuclei by electrical tongue stimulation in balance-impaired individuals. Neuroimage 56:2129–2137. https://doi.org/10.1016/j.neuroimage.2011.03.074
Wildenberg JC, Tyler ME, Danilov YP et al (2011) Electrical tongue stimulation normalizes activity within the motion-sensitive brain network in balance-impaired subjects as revealed by group independent component analysis. Brain Connect 1:255–265. https://doi.org/10.1089/brain.2011.0029
Wildenberg JC, Tyler ME, Danilov YP et al (2013) Altered connectivity of the balance processing network after tongue stimulation in balance-impaired individuals. Brain Connect 3:87–97. https://doi.org/10.1089/brain.2012.0123
Chisholm AE, Malik RN, Blouin JS et al (2014) Feasibility of sensory tongue stimulation combined with task-specific therapy in people with spinal cord injury: a case study. J Neuroeng Rehabil. https://doi.org/10.1186/1743-0003-11-96
Galea MP, Cofré Lizama LE, Bastani A et al (2017) Cranial nerve non-invasive neuromodulation improves gait and balance in stroke survivors: a pilot randomised controlled trial. Brain Stimul 10:1133–1135
Ptito A, Papa L, Gregory K, et al (2020) A prospective, multicenter study to assess the safety and efficacy of translingual neurostimulation plus physical therapy for the treatment of a chronic balance deficit due to mild‐to‐moderate traumatic brain injury. Neuromodulation Technol Neural Interface. https://doi.org/10.1111/ner.13159
Hong S, Quan C-H, Shim H-M, Lee S (2016) Design and implementation of low-power neuromodulation S/W based on MSP430. J Inst Electron Inf Eng 53:110–120. https://doi.org/10.5573/ieie.2016.53.7.110
McCoin JL, Bhadra N, Gustafson KJ (2013) Electrical stimulation of sacral dermatomes can suppress aberrant urethral reflexes in felines with chronic spinal cord injury. Neurourol Urodyn 32:92–97. https://doi.org/10.1002/nau.22276
Raina S (2020) Neuromodulation for restoration of urinary and bowel control. Neurol India 68:307. https://doi.org/10.4103/0028-3886.302457
Sievert KD, Amend B, Gakis G et al (2010) Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol 67:74–84. https://doi.org/10.1002/ana.21814
Stampas A, Gustafson K, Korupolu R et al (2019) Bladder neuromodulation in acute spinal cord injury via transcutaneous tibial nerve stimulation: cystometrogram and autonomic nervous system evidence from a randomized control pilot trial. Front Neurosci 13:119. https://doi.org/10.3389/fnins.2019.00119
Panicker JN, Fowler CJ, Kessler TM (2015) Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol 14:720–732
Ojha R, George J, Chandy BR et al (2015) Neuromodulation by surface electrical stimulation of peripheral nerves for reduction of detrusor overactivity in patients with spinal cord injury: a pilot study. J Spinal Cord Med 38:207–213. https://doi.org/10.1179/2045772313Y.0000000175
Liechti M, Müller R, Lam T, Curt A (2008) Vestibulospinal responses in motor incomplete spinal cord injury. Clin Neurophysiol. https://doi.org/10.1016/j.clinph.2008.05.033
Wydenkeller S, Liechti M, Müller R, Curt A (2006) Impaired scaling of responses to vestibular stimulation in incomplete SCI. Exp Brain Res. https://doi.org/10.1007/s00221-006-0688-z
Čobeljić RD, Ribarič-Jankes K, Aleksić A et al (2018) Does galvanic vestibular stimulation decrease spasticity in clinically complete spinal cord injury? Int J Rehabil Res. https://doi.org/10.1097/MRR.0000000000000297
Lefaucheur JP, Antal A, Ayache SS et al (2017) Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 128:56–92
Gomes-Osman J, Field-Fote EC (2015) Cortical vs. afferent stimulation as an adjunct to functional task practice training: a randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil 29:771–782. https://doi.org/10.1177/0269215514556087
Cortes M, Medeiros AH, Gandhi A et al (2017) Improved grasp function with transcranial direct current stimulation in chronic spinal cord injury. NeuroRehabilitation 41:51–59. https://doi.org/10.3233/NRE-171456
de Araújo AVL, Ribeiro FPG, Massetti T et al (2020) Effectiveness of anodal transcranial direct current stimulation to improve muscle strength and motor functionality after incomplete spinal cord injury: a systematic review and meta-analysis. Spinal Cord 58:635–646
Kumru H, Murillo N, Benito-Penalva J et al (2016) Transcranial direct current stimulation is not effective in the motor strength and gait recovery following motor incomplete spinal cord injury during Lokomat® gait training. Neurosci Lett 620:143–147. https://doi.org/10.1016/j.neulet.2016.03.056
Stimulation to enhance walking post-sci—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03702842. Accessed 17 May 2021
Szecsi J, Fornusek C, Krause P, Straube A (2007) Low-frequency rectangular pulse is superior to middle frequency alternating current stimulation in cycling of people with spinal cord injury. Arch Phys Med Rehabil. https://doi.org/10.1016/j.apmr.2006.12.026
Shapiro S, Borgens R, Pascuzzi R et al (2005) Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine 2:3–10. https://doi.org/10.3171/spi.2005.2.1.0003
Zhang C, Zhang G, Rong W, et al (2014) Oscillating field stimulation promotes spinal cord remyelination by inducing differentiation of oligodendrocyte precursor cells after spinal cord injury. In: Bio-Medical Materials and Engineering. IOS Press, pp 3629–3636
Bacova M, Bimbova K, Fedorova J et al (2019) Epidural oscillating field stimulation as an effective therapeutic approach in combination therapy for spinal cord injury. J Neurosci Methods 311:102–110. https://doi.org/10.1016/j.jneumeth.2018.10.020
Moineau B, Marquez-Chin C, Alizadeh-Meghrazi M, Popovic MR (2019) Garments for functional electrical stimulation: design and proofs of concept. J Rehabil Assist Technol Eng 6:205566831985434. https://doi.org/10.1177/2055668319854340
Kilgore KL, Hoyen HA, Bryden AM et al (2008) An implanted upper-extremity neuroprosthesis using myoelectric control. J Hand Surg Am 33:539–550. https://doi.org/10.1016/j.jhsa.2008.01.007
Memberg WD, Polasek KH, Hart RL et al (2014) Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia. Arch Phys Med Rehabil 95:1201-1211.e1
Chin CA, Barreto A, Cremades JG, Adjouadi M (2008) Integrated electromyogram and eye-gaze tracking cursor control system for computer users with motor disabilities. J Rehabil Res Dev 45:161–174. https://doi.org/10.1682/JRRD.2007.03.0050
Lathem PA, Gregorio TL, Garber SL (1985) High-level quadriplegia: an occupational therapy challenge. Am J Occup Ther Off Publ Am Occup Ther Assoc 39:705–714. https://doi.org/10.5014/ajot.39.11.705
Moreno JC, de Lima ER, Ruíz AF et al (2006) Design and implementation of an inertial measurement unit for control of artificial limbs: application on leg orthoses. Sensors Actuators B Chem 118:333–337. https://doi.org/10.1016/j.snb.2006.04.039
Grishin AA, Bobrova EV, Reshetnikova VV et al (2021) A system for detecting stepping cycle phases and spinal cord stimulation as a tool for controlling human locomotion. Biomed Eng (NY) 54:312–316. https://doi.org/10.1007/s10527-021-10029-7
Fitzgerald MM, Sposato B, Politano P et al (2009) Comparison of three head-controlled mouse emulators in three light conditions. AAC Augment Altern Commun 25:32–41. https://doi.org/10.1080/07434610802640745
Ghovanloo M, Sahadat MN, Zhang Z, et al (2017) Tapping into tongue motion to substitute or augment upper limbs. In: Micro- and nanotechnology sensors, systems, and applications IX. SPIE, p 1019413
Lauer RT, Peckham PH, Kilgore KL (1999) EEG-based control of a hand grasp neuroprosthesis. NeuroReport 10:1767–1771. https://doi.org/10.1097/00001756-199906030-00026
Friedenberg DA, Schwemmer MA, Landgraf AJ et al (2017) Neuroprosthetic-enabled control of graded arm muscle contraction in a paralyzed human. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-08120-9
Bockbrader M, Annetta N, Friedenberg D et al (2019) Clinically significant gains in skillful grasp coordination by an individual with tetraplegia using an implanted brain-computer interface with forearm transcutaneous muscle stimulation. Arch Phys Med Rehabil 100:1201–1217. https://doi.org/10.1016/j.apmr.2018.07.445
Colachis SC, Bockbrader MA, Zhang M et al (2018) Dexterous control of seven functional hand movements using cortically-controlled transcutaneous muscle stimulation in a person with tetraplegia. Front Neurosci 12:208. https://doi.org/10.3389/fnins.2018.00208
Bouton CE, Shaikhouni A, Annetta NV et al (2016) Restoring cortical control of functional movement in a human with quadriplegia. Nature 533:247–250. https://doi.org/10.1038/nature17435
Ajiboye AB, Willett FR, Young DR et al (2017) Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet 389:1821–1830. https://doi.org/10.1016/S0140-6736(17)30601-3
Intelligent spine interface—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04302259. Accessed 17 May 2021
Alizadeh M, Fisher J, Saksena S et al (2018) Reduced field of view diffusion tensor imaging and fiber tractography of the pediatric cervical and thoracic spinal cord injury. J Neurotrauma 35:452–460. https://doi.org/10.1089/neu.2017.5174
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
B.K.—conception, drafting, substantial revision; N.S.—literature review, drafting; B.N.—literature review, drafting; M.S.—literature review, substantial revision; R.H.—substantial revision; J.H.—conception, substantial revision; A.V.—conception, substantial revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karamian, B.A., Siegel, N., Nourie, B. et al. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J Orthop Traumatol 23, 2 (2022). https://doi.org/10.1186/s10195-021-00623-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-021-00623-6