Abstract
Background
Anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies have emerged as promising therapeutic options for the treatment of chronic migraine. However, treatment response varies considerably among individuals, suggesting a potential role for genetic factors. This study aimed to identify genetic variants affecting the efficacy of anti-CGRP monoclonal antibody therapy in chronic migraine among the Han Chinese population in Taiwan to enhance treatment precision and to understand the genetic architecture of migraine.
Methods
We conducted a quantitative trait locus (QTL) association study in patients with chronic migraines from a tertiary medical center in Taiwan using the Taiwan Precision Medicine Array Chip. The patients received fremanezumab or galcanezumab for at least 12 weeks. Treatment efficacy was assessed based on the improvement rate in monthly migraine days. Genetic variants were identified, and their associations with treatment efficacy were examined through quantitative trait loci analysis, linkage disequilibrium studies, and functional annotations using the Gene Ontology database.
Results
Six single nucleotide polymorphisms (SNPs) relative variants were significantly associated with anti-CGRP therapy response (p < 1 × 10− 7): rs116870564, rs75244870, rs56216870, rs12938101, rs74655790, and rs149540851. These variants are located in or near genes, including LRRC4C, ATAD2B, and OXR1, which are involved in neuronal development, DNA-dependent ATPase activity, and oxidation-reduction processes, respectively. The rs116870564 variant in LRRC4C showed the strongest association (β = -0.551, p = 6.65 × 10− 9). The functional impact of these variants is attributed to their regulatory effects on gene expression, which are influenced by intron splicing regulation, transcription factors, and changes in chromatin structure.
Conclusion
The identification of key genetic markers associated with response to anti-CGRP therapy emphasizes the importance of genetic variability in treatment efficacy. This could lead to more personalized chronic migraine management strategies and tailored therapeutic approaches based on individual genetic profiles. Further research in larger, diverse populations is warranted to validate these findings and refine our understanding of the role of CGRP in chronic migraine pathophysiology.
Trial registration
Not applicable.
Similar content being viewed by others
Background
Migraine is a significant global health challenge, acknowledged among the most disabling diseases worldwide [1]. It is characterized by severe episodic headaches frequently accompanied by nausea, vomiting, and heightened sensitivity to light and sound, significantly impacting quality of life [2]. Chronic migraine (CM), its severe subtype, involves headaches on 15 or more days per month for at least three months, with migraine features present on at least eight days monthly [3]. The frequent and intense headaches impair daily functioning and productivity, marking CM as a major public health concern [4]. Globally, CM affects 1–2% of the general population, imposing substantial burdens on individuals, healthcare systems, and society [5, 6]. In the Asia-Pacific region, including Taiwan, migraine prevalence is estimated at 9.1%, underlining its local significance [7].
Migraine pathophysiology is complex and multifactorial, influenced by genetic and environmental factors. Recent genome association studies have identified over 100 loci associated with migraine susceptibility, highlighting its polygenic nature [8, 9]. These genetic findings provide insights into biological pathways, such as neurotransmitter signaling, vascular function, and ion channel activity [10]. Calcitonin gene-related peptide (CGRP) plays a pivotal role in migraine pathophysiology, acting within the trigeminovascular system [11]. CGRP, a potent vasodilator released during migraine attacks, facilitates pain transmission, making it a prime therapeutic target. Monoclonal antibodies targeting CGRP or its receptors (e.g., erenumab, fremanezumab, galcanezumab, and eptinezumab) have emerged to reduce migraine attack frequency [12, 13], marking significant advances in migraine management.
Despite the general efficacy of CGRP monoclonal antibodies, substantial variations exist in treatment responses among patients. Clinical trials show some achieving up to 75% reduction or complete migraine freedom [14]. However, response rates generally range from 40–70% [15], highlighting the need to explore factors influencing treatment efficacy. Pharmacogenomics, which studies genetic influences on drug responses, has the potential to personalize migraine treatments and improve patient outcomes. Results of large-scale genome-wide association studies (GWAS) revealed specific single nucleotide polymorphisms (SNPs) within CGRP-related genes, suggesting genetic variations increase migraine risk and modify responses to CGRP antagonists [8, 9].
Known genetic associations in migraine include variants in genes for neurotransmitter systems (e.g., MTDH and PRDM16), ion channels (e.g., KCNK5 and TRPM8), and vascular functions (e.g., PHACTR1 and FHL5) [8, 9]. These findings suggest genetic factors impacting migraine susceptibility may also influence treatment responses. Additionally, variants in CGRP signaling genes, such as CALCA (encoding CGRP-Alpha) and RAMP1 (encoding a component of the CGRP receptor), could be particularly relevant for anti-CGRP therapy outcomes [16]. A recent retrospective study highlighted clinical and genetic factors influencing responses to anti-CGRP monoclonal antibody therapy, with variations in genes, such as RAMP1 linked to varying response rates.
Most genetic migraine studies focus on European ancestry populations, leaving a significant gap for other ethnic groups, including Asian populations [17]. Considering the known variations in migraine prevalence and clinical presentation across ethnicities, it is necessary to investigate genetic factors influencing treatment responses across diverse populations [18]. The Han Chinese population, the largest ethnic group in East Asia, remains underrepresented in migraine genetic studies, particularly regarding anti-CGRP therapy responses. Taiwan, with its advanced healthcare system and relatively homogeneous Han Chinese population, provides an ideal setting to investigate genetic influences on treatment responses in this ethnic group [19]. Therefore, we aim to identify susceptibility loci associated with anti-CGRP therapy efficacy for CM in the Han Chinese population of Taiwan. By identifying genetic variants affecting responses to anti-CGRP monoclonal antibody therapy, we aim to refine treatments and enhance outcomes for patients with migraines within this demographic.
Materials and methods
Study design and participants
We conducted a prospective, observational genetic study involving a cohort of patients with CM recruited from the neurology outpatient department of a tertiary medical center in Taiwan. This study combined clinical outcome data with genetic analysis to investigate associations between genetic variants and treatment response. The study cohort comprised individuals diagnosed with CM treated with galcanezumab (Emgality) or fremanezumab (Ajovy). The study protocol was approved by the Institutional Review Board of the Tri-Service General Hospital, and all participants provided written informed consent prior to enrollment. Inclusion criteria were: (1) age ≥ 18 years, (2) diagnosis of CM according to the criteria in the third edition of the International Classification of Headache Disorders (ICHD-3) [3], and (3) Han Chinese ethnicity. The exclusion criterion was secondary or other concomitant primary headache disorders.
Each participant completed a screening questionnaire and was interviewed by a board-certified neurologist and headache specialist (FCY). All patients completed a standardized demographic questionnaire and the Migraine Disability Assessment questionnaire (MIDAS) [20]. Documented clinical characteristics included sex, age, aura symptoms, medication overuse, triptan response, preventive treatment failure (drug class), body mass index, education (years), migraine duration (years), monthly migraine days (MMD), monthly headache days (MHD), MIDAS score, and improvement rate after treatment with anti-CGRP monoclonal antibody (Table 1).
Anti-CGRP therapy protocol
Galcanezumab was administered at a loading dose of 240 mg, followed by a monthly subcutaneous injection of 120 mg. Fremanezumab was administered as monthly 225 mg subcutaneous injections or quarterly 675 mg injections based on individual patient needs. Treatment was administered for 12 weeks, with follow-up visits scheduled at 4-week intervals.
Genotyping by TPM array
DNA extraction and identification of nucleotide mutations were performed as follows: (1) DNA extraction: genomic DNA was extracted and purified from 3 mL of peripheral blood collected in EDTA vacutainers using a QIAsymphony SP system (QIAGEN, Hilden, Germany); (2) genotyping: purified DNA from each participant was loaded onto a Taiwan Precision Medicine (TPM) array chip and genome type signals were detected using the Axiom GeneTitan platform (Thermo Fisher Scientific, Sunnyvale, CA, USA) [21]; and (3) quality control and annotation: quality control of the genome SNP data, SNP calling, and sample annotations were performed using the Axiom Analysis Suite (Thermo Fisher Scientific).
Quantitative trait locus (QTL) analysis
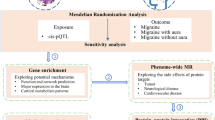
We analyzed genotyping data from the TPM array [21]. SNPs (493, 292 in total) with a low typing call rate (< 80%) were excluded. To characterize the relationship between the improvement rate and probability risk SNPs (variants), QTL with a linear regression model [22] was applied using PLINK 1.9 software [23]. Improvement rate values were used as the QTL phenotype, and clinical factors, such as sex, age, aura, MMD, MHD, medication overuse, triptan response, preventive treatment failure, migraine duration, and MIDAS score were tested as covariates in the linear regression. After systematically arranging and combining different covariates to explore all possible permutations and combinations, suitable combinations with the most significant variants were used for further interpretation. Figure 1 outlines the steps involved in QTL analysis.
Study Flowchart. This flowchart outlines the steps involved in this study, including participant recruitment and data analysis. The workflow included genotyping using the Taiwan Precision Medicine array, quantitative trait locus analysis, and subsequent steps, such as Gene Ontology enrichment and linkage disequilibrium analysis. Key elements include SNP filtering, application of the linear regression model using PLINK 1.9 software, and systematic exploration of covariate combinations to identify significant variants associated with the anti-CGRP monoclonal antibody therapy response in patients with chronic migraine
Variant annotations and functional analysis
Significant variants and their associated genes were annotated using the RefSeq Database (https://www.ncbi.nlm.nih.gov/refseq/) from wANNOVAR (https://wannovar.wglab.org/) [24]. Allele frequencies across diverse populations were assessed using the 1000 Genomes Project [25] and Taiwan BioBank [26]. Gene functional characterization was performed using Gene Ontology (GO) [27], and GO enrichment analysis was conducted using DAVID v6.8 [28] to identify biological processes, associated with genes harboring or near significant SNPs.
Linkage disequilibrium (LD) analysis
LD analysis was conducted to understand which genes were likely to be functionally relevant to the trait or disease as well as the number and location of contributing genes [29]. We uploaded the intronic variants rs116870564, rs74655790, and rs149540851, obtained from QTL analysis, using the LDproxy Tool, part of LDlink (https://ldlink.nih.gov/?tab=home) [30, 31]. The analysis parameters were as follows: - Genome build version: GRCh38 - Populations analyzed: CDX (Chinese Dai in Xishuangbanna, China), CHB (Han Chinese in Beijing, China), and CHS (Southern Han Chinese) - LD measurements: D’ (D prime) and R² (R-squared), calculated based on allele frequencies - Base pair window: 10,000 bp - Regulatory potential predictions: FORGEdb [32]. The 10,000-bp window size was chosen to capture a wide range of potential regulatory elements and interactions, providing a detailed map of the genomic landscape surrounding the variants.
Statistical analysis
Demographic and clinical characteristics were compared between treatment groups using independent two-sample t-tests for continuous variables and chi-squared tests for categorical variables. The significance threshold was set at p < 1 × 10− 7 and results were visualized using CMPlot [33] for circular Manhattan and Q-Q plots to assess potential genomic inflation. All statistical analyses were performed using R version 4.0.3 [34]. Statistical significance was defined as a two-sided p-value of < 0.05, unless otherwise specified.
We chose a significance threshold of p < 1 × 10⁻⁷ to address the multiple comparisons involved in analyzing 493,292 SNPs and ensure robustness against false positives. This threshold is slightly more conservative than the standard p < 5 × 10⁻⁸ typically used in GWAS, due to the specific design considerations of our study, including the use of the Taiwan Precision Medicine Array Chip and the sample size. This approach balances the need to identify true associations with the control of type I error rates.
Results
Participant demographics and clinical characteristics
A total of 108 Han Chinese patients with CM were enrolled in this study. The cohort comprised 89 females (82.4%) and 19 males (17.6%), with a mean age of 46.8 ± 14.83 years. Participants were divided into two treatment groups: 40 patients (37%) received fremanezumab, and 68 patients (63%) received galcanezumab. Table 1 shows the demographic and clinical characteristics of the study population. Most patients (91.7%) reported medication overuse, and 74.1% were triptan responders. Migraine with aura was present in 20.4% of participants. The mean duration of migraine history was 26.8 ± 13.1 years. At baseline, patients experienced an average of 22.19 ± 7.3 MMDs and 24.55 ± 5.86 MHDs. The mean MIDAS score was 32.2 ± 17.9, indicating severe disability. There were no statistically significant differences between the fremanezumab and galcanezumab groups in demographic or clinical characteristics (all p > 0.05), suggesting that the two treatment groups were comparable at baseline.
Treatment efficacy overview
The overall mean improvement rate in monthly migraine days after 12 weeks of anti-CGRP monoclonal antibody treatment was 78.93 ± 18.27%. There was no significant difference in improvement rates between the fremanezumab (78.25 ± 16.27%) and galcanezumab (79.33 ± 19.45%) groups (p = 0.767).
Significant genetic variants identified
QTL association analysis identified six SNPs significantly associated with anti-CGRP therapy response (p < 1 × 10− 7). These variants are presented in Table 2 and visualized using a Manhattan plot (Fig. 2A) and a Q-Q plot (Fig. 2B). The most significant association was observed for rs116870564 (p = 6.65 × 10− 9), an intronic variant in the LRRC4C gene on chromosome 11. This variant showed a strong negative effect on treatment response (β = -0.551, SE = 0.087), indicating that the minor allele is associated with a poorer response to anti-CGRP therapy.
Quantitative trait locus (QTL) analysis results for anti-CGRP monoclonal antibody therapy response in chronic patients with migraines. (A) Circular Manhattan plot showing the QTL association results. Each point represents a SNP, with its position on the respective chromosome indicated by the outer circle. The inner circle shows the statistical significance (-log10 p-value) of each SNP’s association with treatment response. The blue dashed line indicates the threshold for significance (p < 1 × 10− 7). Six highly significant variants are highlighted and labeled: rs116870564, rs75244870, rs56216870, rs12938101, rs74655790, and rs149540851. (B) Quantile-Quantile (Q-Q) plot of the observed versus expected -log10 p-values from the association analysis. The red line represents the null hypothesis of no association. Deviations above this line indicate potential true genetic associations with the improvement rate. The genomic inflation factor (λ) is provided, calculated based on the median of observed and expected chi-square values, demonstrating the robustness of the test statistics
Other significant variants included rs75244870 (p = 6.92 × 10− 9) near acyl-CoA oxidase 2 (ACOX2) on chromosome 3, rs56216870 (p = 1.77 × 10− 8) near metastasis suppressor 1 (MTSS1) on chromosome 8, rs12938101 (p = 3.85 × 10− 8) near TMEM92-AS1 on chromosome 17, rs74655790 (p = 4.24 × 10− 8) in ATAD2B on chromosome 2, and rs149540851 (p = 4.24 × 10− 8) in OXR1 on chromosome 8. The Q-Q plot (Fig. 2B) revealed significant deviations above the diagonal line, indicating a true association of potential genetic link to the improvement rate. The genomic inflation factor (lambda) was close to one, suggesting minimal inflation and confirming the robustness of the test statistics. Notably, when the galcanezumab and fremanezumab cohorts were analyzed separately, only one significant variant (rs141304376) was detected in the galcanezumab group, and no significant variants were found in the fremanezumab group (Supplementary Fig. 1).
While medication overuse was prevalent among around 90% of our participants, none met the formal diagnostic criteria for medication overuse headache (MOH) as defined by the ICHD-3. Therefore, these patients were not excluded from the study. An exploratory analysis was conducted to assess potential genetic associations with medication overuse, despite these patients not meeting full MOH criteria [35]. However, no statistically significant variants were identified in this regard (Supplementary Table 1). This analysis included 33 MOH-associated genes, of which 18 had SNPs available on the TPM array used in our study.
Genotype-phenotype associations
Figure 3 illustrates the associations between the genotypes of the six significant SNPs and the improvement rates on monthly migraine days. For rs116870564, individuals with the GG genotype (n = 105) showed a significantly higher mean improvement rate (0.83 ± 0.15) compared to those with the AG genotype (n = 2, mean improvement rate 0.12 ± 0.04, p < 0.001). Similar trends were observed for other significant variants. Notably, the heterozygous rs116870564, rs74655790, and rs149540851 genotypes showed a strong decrease in improvement rates (up to 70%). The variant rs56216870 displayed an interesting pattern, with a more pronounced effect in the homozygous mutant genotype than in the heterozygous genotype.
Genotype-phenotype associations for significant variants identified through quantitative trait locus analysis. Box plots showing the association between the genotypes of six significant SNPs (rs116870564, rs75244870, rs56216870, rs12938101, rs74655790, and rs149540851) and improvement rates in monthly migraine days after anti-CGRP monoclonal antibody therapy. For each variant, the distribution of improvement rates is shown for the different genotypes. The heterozygous mutations AG, TC, and AC in rs116870564, rs74655790, and rs149540851, respectively, and the homozygous mutation TT in rs56216870 showed a strong decrease in improvement rates compared to the normal genotypes. Boxes represent the interquartile range, with the median indicated by a horizontal line. Whiskers extend to minimum and maximum values, excluding outliers
Analysis of individual participants revealed that some, such as participants 94 and 1, harbored multiple variants associated with poor improvement rates (Table 3). Most participants with poor improvement rates also showed poor response to triptan, which reduces CGRP release.
Further analysis of potential covariates, including sex, age, aura, MMD, MHD, medication overuse, migraine duration, and MIDAS score revealed that triptan response was the most significant covariate (p < 0.05) in the regression model for most variants. Supplementary Fig. 2 illustrates the participants with poor triptan response also tended to have poor improvement (≤ 0.25, below the lower quartile) with anti-CGRP monoclonal antibody therapy. Supplementary Table 2 presents the positive effect (BETA values) of triptan response on the six significant variants. Notably, given that the variant effects are negative, these findings suggest that certain genetic factors may contribute to reduced efficacy of both triptan and anti-CGRP therapies, potentially through shared mechanistic pathways.
LD and functional annotation results
LD analysis was performed to investigate the genomic context of significant variants. Figure 4 shows LD plots for the three most significant loci: LRRC4C (Fig. 4A), ATAD2B (Fig. 4B), and OXR1 (Fig. 4C). The lead SNP, rs116870564, in LRRC4C showed moderate LD with several nearby variants, suggesting a potential regulatory region in intron. Similarly, rs74655790 in ATAD2B and rs149540851 in OXR1 were found to be in LD with other intronic variants in their respective genes.
Linkage disequilibrium (LD) analysis of intronic variants. LD plots and functional annotations for the three significant intronic variants: (A) rs116870564 in LRRC4C (chromosome 11). (B) rs74655790 in ATAD2B (chromosome 2). (C) rs149540851 in OXR1 (chromosome 8). Each panel shows the LD structure around focal SNP. The color gradient, ranging from white to deep blue and red, visualizes the spectrum of D’ (D prime) and R² (R-squared) values spanning from 0.0 to 1.0 in the relative chromosome region. White numbers in the purple squares represent FORGEdb scores, indicating the likelihood of variants functioning as regulatory elements. The red text indicates the query intronic variants from the quantitative trait locus analysis. The genes in this region are shown below each plot and thin lines represent intron regions and bold lines indicate exon regions
Functional annotation revealed that most significant SNPs were located in intronic or intergenic regions. The FORGEdb scores predict the likelihood of genetic variants functioning as regulatory elements (Fig. 4). The variants associated with rs116870564 and rs74655790 and their LD associated variants showed moderate regulatory effects on LRRC4C and ATAD2B, respectively. Notably, variants in LD with rs149540851 demonstrated strong regulatory effects on OXR1, suggesting additional genetic risks outside the protein-coding regions.
GO enrichment analysis findings
GO enrichment analysis results revealed biological processes associated with genes harboring or near significant SNPs (Table 4). LRRC4C, containing the intronic variant rs116870564, is involved in critical synaptic functions, such as axonogenesis (GO:0050770) and synaptic membrane adhesion (GO:0099560). ACOX2 is associated with metabolic pathways, such as bile acid and fatty acid biosynthesis. FAM107A is associated with cell adhesion and responses to nutrients. MTSS1 is involved in actin filament dynamics and cellular structural processes. XYLT2 was found to be crucial for glycosaminoglycan biosynthesis, affecting extracellular matrix formation, and potentially modulating cellular interactions.
Discussion
This study of a Taiwanese CM cohort using the TPM array chip represents the first genome association study to investigate the genetic determinants of anti-CGRP monoclonal antibody therapy response in a Han Chinese population. Our analysis of 108 patients identified six SNPs significantly associated with treatment response: rs116870564 in LRRC4C, rs75244870 near ACOX2, rs56216870 near MTSS1, rs12938101 near TMEM92-AS1, rs74655790 in ATAD2B, and rs149540851 in OXR1. These variants were strongly associated with reduced improvement rates following anti-CGRP therapy, suggesting their potential role in modulating treatment efficacy.
Our findings contribute to the growing body of evidence supporting the role of genetic factors in migraine pathophysiology and treatment responses. Previous GWASs have identified numerous loci associated with migraine susceptibility [8, 9]; however, research on the genetic determinants of anti-CGRP therapy response has been limited. The identification of novel variants in our study highlights the importance of investigating treatment responses in diverse populations, as the genetic architecture may vary across ethnicities. The most significant variant identified in our study, rs116870564 in LRRC4C (leucine rich repeats containing 3 C), has not been previously reported in migraine-related studies. However, LRRC4C are known to play a role in axon guidance and synaptic plasticity [36], which are processes that have been implicated in migraine pathophysiology [37]. This finding aligns with the growing understanding that migraine is a complex neurological disorder involving alterations in neuronal excitability and synaptic function.
The identification of variants near ACOX2 and MTSS1 is notable because these genes have not been directly linked to migraine in previous studies. ACOX2 is involved in fatty acid metabolism [38], while MTSS1 plays a role in actin dynamics and cellular structure [39]. These associations suggest potential novel pathways involved in migraine pathophysiology or treatment responses that warrant further investigation. The variant rs74655790 in ATAD2B is of particular interest, as ATAD2B has been implicated in chromatin remodeling and transcriptional regulation [40]. This finding supports the emerging concept that epigenetic mechanisms play a role in migraine susceptibility and treatment response [41].
The identified genetic variants and their associated genes provide insights into the potential biological mechanisms underlying the variability in response to anti-CGRP therapy. We propose several hypotheses based on our findings and the current understanding of migraine pathophysiology: (1) Neuronal Plasticity and Synaptic Function: LRRC4C, which encodes netrin-G ligand-1 (NGL-1), is a post-synaptic adhesion molecule integral to the formation, development, and modulation of synaptic connections, affecting neurotransmitter signaling, neuronal network integrity, and axonogenesis [42]. CGRP has been shown to play a crucial role in synaptic remodeling and axonal sprouting, particularly at neuromuscular junctions [43]. The LRRC4C variant may influence these processes, potentially affecting neuronal circuits involved in pain processing and migraine pathophysiology. Alterations in these processes could modulate the response to the CGRP signaling blockade, explaining the observed variability in treatment efficacy [44]. (2) Hippocampal Function and Pain Processing: LRRC4C affects the hippocampus, a brain region critical for learning, memory, and emotional regulation, by affecting synaptic density and plasticity [45, 46]. The hippocampus plays a key role in pain-related attention, anxiety, and stress responses through its connection to the hypothalamic-pituitary-adrenal axis [47]. These functions suggest a potential role in the pathophysiology of migraines, possibly influencing the threshold for migraine initiation, intensity of pain, and cognitive symptoms associated with migraines [48, 49]. (3) Chromatin Remodeling and Transcriptional Regulation: ATAD2B plays an important role in chromatin remodeling and transcriptional regulation, which are essential for cellular development and differentiation, especially in neurons [50]. Although not directly linked to migraines, its involvement in critical cellular functions and its observation in various brain tumors [51] suggest that epigenetic mechanisms may influence the response to anti-CGRP therapy. Chromatin remodeling and transcriptional regulation can affect the expression of genes involved in CGRP signaling and pain processing, leading to variability in treatment outcomes [41]. (4) Oxidative Stress Response: The variant in OXR1 implicates oxidative stress pathways in migraine treatment response. OXR1 plays a critical role in protecting cells from oxidative damage, which is vital for maintaining cellular health and preventing apoptosis, particularly in neurons [52]. Oxidative stress is associated with migraine pathophysiology [53], and variations in antioxidant mechanisms can modulate the effectiveness of anti-CGRP therapy [54, 55]. By stabilizing mitochondrial function and reducing oxidative damage in neurons, OXR1 may help modulate neuronal excitability and inflammatory responses, which are often heightened in patients with migraine. (5) Metabolic Pathways: The association with ACOX2 suggests a potential link between fatty acid metabolism and migraines. ACOX2 encodes peroxisomal branched-chain acyl-CoA oxidase, which is key to bile acid and fatty acid biosynthesis and affecting brain inflammation [56]. Lipid metabolism has been implicated in neuroinflammation and pain signaling [57], and variations in these pathways can influence the effectiveness of anti-CGRP therapy by altering the local tissue environment or modulating CGRP receptor function. (6) Cytoskeletal Dynamics: The variant near MTSS1 suggests a possible role for actin dynamics and cellular structure in migraine pathophysiology. MTSS1 is essential for maintaining cellular integrity in kidney epithelia and neuronal structures and regulating plasma membrane dynamics and actin filament assembly [58]. Cytoskeletal remodeling is crucial for neuronal function and synaptic plasticity [59], and alterations in these processes can affect responses to CGRP signaling.
The identification of genetic variants that influence the efficacy of anti-CGRP monoclonal antibody therapy offers significant clinical implications, particularly in the context of personalized medicine. As highlighted in a recent review [60], the genetic profile of patients with migraines plays a key role in determining their response to various treatments. Our findings suggest that genetic screening could be incorporated into clinical practice to identify patients who are more likely to benefit from CGRP-targeted therapies. For example, the SNP rs116870564 in the LRRC4C gene was associated with a lower improvement rate, indicating that patients carrying this variant may require alternative therapeutic approaches or dosage adjustments. Our findings suggest that genetic profiling can potentially be used to predict treatment outcomes and guide therapeutic decisions. For instance, patients carrying variants associated with a poor response to anti-CGRP therapy may benefit from alternative treatment strategies or combination therapies. Moreover, the discovery of novel genes and pathways involved in the treatment response opens up possibilities for the development of new therapeutic targets.
The observed association between a poor response to anti-CGRP therapy and to triptans in some patients suggests a potential shared mechanism of treatment resistance, which aligns with previous studies indicating a correlation between triptan responses and responses to anti-CGRP treatments [61, 62]. This correlation may be attributed to overlapping molecular pathways, such as those involving serotonin and CGRP signaling in the trigeminovascular system [63, 64]. Some studies have also explored the effects of genetic variants on the efficacy of triptans, showing significant correlations for certain polymorphisms [65, 66]. Future studies should include elucidation of the specific molecular mechanisms underlying this observed correlation, which could inform more personalized treatment strategies for patients with CM. These insights align with the broader movement towards precision medicine, where treatments are tailored to the genetic profiles of individual patients, thereby optimizing efficacy and reducing the trial-and-error approach in migraine management. As the field progresses, the integration of pharmacogenomic data into routine clinical practice will be essential for enhancing patient care and improving the quality of life for with CM. Furthermore, although our exploratory analysis did not reveal significant genetic variants associated with medication overuse in this cohort, this area warrants further investigation. This negative finding might be due to our sample size, the specific genetic markers examined, or the complex nature of medication overuse in CM. Larger studies focusing specifically on MOH may be required to investigate genetic factors contributing to this challenging clinical phenomenon.
Strengths: First, this study is the first genome association study to investigate the response to anti-CGRP therapy in a Han Chinese population, addressing an important gap in the literature. Second, the use of a homogeneous population reduced the potential confounding effects of population stratification. Third, a comprehensive approach, including functional annotation and pathway analysis, provides insights into the potential biological mechanisms. Fourth, the identification of rare variants with low allele frequencies in Taiwanese and East Asian populations suggests the presence of ancestral genetic alleles that may harbor functionally important elements affecting the molecular mechanisms of headache. Limitations: First, the sample size, while substantial for a pharmacogenomic study in a specific population, was relatively modest for genome association study. Second, our study relied on a 12-week follow-up period to assess treatment efficacy. Recent research has highlighted the potential discrepancies between short-term and long-term outcomes in patients undergoing anti-CGRP monoclonal antibody therapy. For example, a multicenter study demonstrated poor agreement between 3- and 12-month response rates, suggesting that early response may not reliably predict sustained treatment efficacy over time [67]. This finding emphasizes the need for extended follow-up periods in future studies to fully understand the long-term impact of these therapies on CM. Additionally, the study identified clinical factors, such as continuous headache at baseline and the number of previously trialed preventive treatments, as potential predictors of long-term response, which should be considered in future research.
Conclusions
Our study highlights the significant role of genetic variations in influencing the response to anti-CGRP monoclonal antibody therapy in patients with CM in the Han Chinese population. We identified six genetic variants located in or near the genes, LRRC4C, ACOX2, MTSS1, TMEM92-AS1, ATAD2B, and OXR1 that were significantly associated with treatment response. These findings offer new insights into the biological mechanisms underlying migraine pathophysiology and treatment response, emphasizing the potential roles of synaptic plasticity, oxidative stress, and metabolic pathways in modulating the efficacy of anti-CGRP therapy. By identifying key genetic markers associated with treatment outcomes, we provide a foundation for personalized migraine management. These results emphasize the potential for tailoring therapeutic strategies based on individual genetic profiles, potentially improving treatment efficacy and patient outcomes. This approach aligns with the growing trend towards precision medicine for neurological disorders. Moreover, the findings of this study emphasize the necessity for further investigations to validate these genetic markers across larger and more ethnically diverse populations. Future research should also explore the long-term implications of these genetic associations and their potential to guide the development of new therapeutic targets.
Data availability
No datasets were generated or analysed during the current study.
References
Collaborators GBDH (2018) Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Neurol 17:954–976
Steiner TJ, Stovner LJ, Vos T (2016) GBD 2015: migraine is the third cause of disability in under 50s. J Headache Pain 17:104
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. (2018) ;38:1-211
Buse DC, Manack A, Serrano D, Turkel C, Lipton RB (2010) Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 81:428–432
Natoli JL, Manack A, Dean B et al (2010) Global prevalence of chronic migraine: a systematic review. Cephalalgia 30:599–609
Blumenfeld AM, Varon SF, Wilcox TK et al (2011) Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia 31:301–315
Peng KP, Wang SJ (2014) Epidemiology of headache disorders in the Asia-Pacific region. Headache 54:610–618
Gormley P, Anttila V, Winsvold BS et al (2016) Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 48:856–866
Hautakangas H, Winsvold BS, Ruotsalainen SE et al (2022) Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet 54:152–160
Eising E, de Vries B, Ferrari MD, Terwindt GM, van den Maagdenberg AM (2013) Pearls and pitfalls in genetic studies of migraine. Cephalalgia 33:614–625
Edvinsson L (2017) The Trigeminovascular pathway: role of CGRP and CGRP receptors in Migraine. Headache 57(Suppl 2):47–55
Silberstein SD, Dodick DW, Bigal ME et al (2017) Fremanezumab for the Preventive treatment of chronic migraine. N Engl J Med 377:2113–2122
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR (2018) Evaluation of Galcanezumab for the Prevention of episodic migraine: the EVOLVE-1 Randomized Clinical Trial. JAMA Neurol 75:1080–1088
Tepper SJ, Ashina M, Reuter U et al (2020) Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia 40:543–553
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK (2018) Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology 91:e2211–e2221
Sutherland HG, Griffiths LR (2017) Genetics of Migraine: insights into the molecular basis of Migraine disorders. Headache 57:537–569
Chasman DI, Schurks M, Anttila V et al (2011) Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet 43:695–698
Robbins MS, Lipton RB (2010) The epidemiology of primary headache disorders. Semin Neurol 30:107–119
Fan CT, Lin JC, Lee CH (2008) Taiwan Biobank: a project aiming to aid Taiwan’s transition into a biomedical island. Pharmacogenomics 9:235–246
Stewart WF, Lipton RB, Dowson AJ, Sawyer J (2001) Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 56:S20–28
Wei CY, Yang JH, Yeh EC et al (2021) Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom Med 6:10
Goddard ME, Hayes BJ (2009) Mapping genes for complex traits in domestic animals and their use in breeding programmes. Nat Rev Genet 10:381–391
Purcell S, Neale B, Todd-Brown K et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575
Yang H, Wang K (2015) Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat Protoc 10:1556–1566
Genomes Project C, Auton A, Brooks LD et al (2015) A global reference for human genetic variation. Nature 526:68–74
Feng YA, Chen CY, Chen TT et al (2022) Taiwan Biobank: a rich biomedical research database of the Taiwanese population. Cell Genom 2:100197
Carbon S, Ireland A, Mungall CJ et al (2009) AmiGO: online access to ontology and annotation data. Bioinformatics 25:288–289
Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Neale BM (2010) Introduction to linkage disequilibrium, the HapMap, and imputation. Cold Spring Harb Protoc. ;2010:pdb top74
Machiela MJ, Chanock SJ (2015) LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31:3555–3557
Machiela MJ, Chanock SJ (2018) LDassoc: an online tool for interactively exploring genome-wide association study results and prioritizing variants for functional investigation. Bioinformatics 34:887–889
Breeze CE, Haugen E, Gutierrez-Arcelus M et al (2024) FORGEdb: a tool for identifying candidate functional variants and uncovering target genes and mechanisms for complex diseases. Genome Biol 25:3
Yin L, Zhang H, Tang Z et al (2021) rMVP: a Memory-efficient, Visualization-enhanced, and parallel-accelerated Tool for Genome-wide Association study. Genomics Proteom Bioinf 19:619–628
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Cargnin S, Viana M, Sances G, Tassorelli C, Terrazzino S (2018) A systematic review and critical appraisal of gene polymorphism association studies in medication-overuse headache. Cephalalgia 38:1361–1373
de Wit J, Ghosh A (2016) Specification of synaptic connectivity by cell surface interactions. Nat Rev Neurosci 17:22–35
Boran HE, Bolay H (2013) Pathophysiology of Migraine. Noro Psikiyatr Ars 50:S1–S7
Ferdinandusse S, Denis S, Mooyer PA et al (2006) Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann Neurol 59:92–104
Saarikangas J, Mattila PK, Varjosalo M et al (2011) Missing-in-metastasis MIM/MTSS1 promotes actin assembly at intercellular junctions and is required for integrity of kidney epithelia. J Cell Sci 124:1245–1255
Morozumi Y, Boussouar F, Tan M et al (2016) Atad2 is a generalist facilitator of chromatin dynamics in embryonic stem cells. J Mol Cell Biol 8:349–362
Eising E, van den Maagdenberg NAD, Ferrari AM (2013) Epigenetic mechanisms in migraine: a promising avenue? BMC Med 11:26
Choi Y, Park H, Kang S et al (2019) NGL-1/LRRC4C-Mutant mice display hyperactivity and Anxiolytic-Like Behavior Associated with widespread suppression of neuronal activity. Front Mol Neurosci 12:250
Tarabal O, Caldero J, Ribera J et al (1996) Regulation of motoneuronal calcitonin gene-related peptide (CGRP) during axonal growth and neuromuscular synaptic plasticity induced by botulinum toxin in rats. Eur J Neurosci 8:829–836
Noseda R, Burstein R (2013) Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 154(Suppl 1):S44–53
Choi Y, Park H, Jung H et al (2019) NGL-1/LRRC4C deletion moderately suppresses hippocampal excitatory synapse development and function in an input-independent manner. Front Mol Neurosci 12:119
Lucassen PJ, Pruessner J, Sousa N et al (2014) Neuropathology of stress. Acta Neuropathol 127:109–135
Liu MG, Chen J (2009) Roles of the hippocampal formation in pain information processing. Neurosci Bull 25:237–266
Liu HY, Chou KH, Chen WT (2018) Migraine and the Hippocampus. Curr Pain Headache Rep 22:13
Yang S, Chang MC (2019) Chronic Pain: structural and functional changes in Brain structures and Associated negative Affective States. Int J Mol Sci ;20
Leachman NT, Brellier F, Ferralli J, Chiquet-Ehrismann R, Tucker RP (2010) ATAD2B is a phylogenetically conserved nuclear protein expressed during neuronal differentiation and tumorigenesis. Dev Growth Differ 52:747–755
Liu H, Wen Q, Yan S et al (2022) Tumor-promoting ATAD2 and its preclinical challenges. Biomolecules ;12
Matsui A, Hashiguchi K, Suzuki M, Zhang-Akiyama QM (2020) Oxidation resistance 1 functions in the maintenance of cellular survival and genome stability in response to oxidative stress-independent DNA damage. Genes Environ 42:29
Togha M, Razeghi Jahromi S, Ghorbani Z, Ghaemi A, Rafiee P (2019) An investigation of oxidant/antioxidant balance in patients with migraine: a case-control study. BMC Neurol 19:323
Durham PL (2016) Diverse physiological roles of calcitonin gene-related peptide in Migraine Pathology: modulation of neuronal-glial-Immune cells to promote Peripheral and Central Sensitization. Curr Pain Headache Rep 20:48
Özge ABB, Bıçakçı Ş, Ertaş M, Atalar AÇ, Gümrü S, Karlı N (2024) Revolutionizing migraine management: advances and challenges in CGRP-targeted therapies and their clinical implications. Front Neurol 15:1402569
Vilarinho S, Sari S, Mazzacuva F et al (2016) ACOX2 deficiency: a disorder of bile acid synthesis with transaminase elevation, liver fibrosis, ataxia, and cognitive impairment. Proc Natl Acad Sci U S A 113:11289–11293
Piomelli D, Sasso O (2014) Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci 17:164–174
Dawson JC, Bruche S, Spence HJ, Braga VM, Machesky LM (2012) Mtss1 promotes cell-cell junction assembly and stability through the small GTPase Rac1. PLoS ONE 7:e31141
Hotulainen P, Hoogenraad CC (2010) Actin in dendritic spines: connecting dynamics to function. J Cell Biol 189:619–629
Tsirelis D, Tsekouras A, Stamati P et al (2024) The impact of genetic factors on the response to migraine therapy. Rev Neurosci
Raffaelli B, Fitzek M, Overeem LH, Storch E, Terhart M, Reuter U (2023) Clinical evaluation of super-responders vs. non-responders to CGRP(-receptor) monoclonal antibodies: a real-world experience. J Headache Pain 24:16
Frattale I, Caponnetto V, Casalena A et al (2021) Association between response to triptans and response to erenumab: real-life data. J Headache Pain 22:1
Christensen CE, Younis S, Deen M, Khan S, Ghanizada H, Ashina M (2018) Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. J Headache Pain 19:105
Frederiksen SD, Haanes KA, Warfvinge K, Edvinsson L (2019) Perivascular neurotransmitters: regulation of cerebral blood flow and role in primary headaches. J Cereb Blood Flow Metab 39:610–632
Gentile G, Borro M, Lala N, Missori S, Simmaco M, Martelletti P (2010) Genetic polymorphisms related to efficacy and overuse of triptans in chronic migraine. J Headache Pain 11:431–435
Cargnin S, Viana M, Sances G, Cantello R, Tassorelli C, Terrazzino S (2019) Using a genetic risk score Approach to Predict Headache response to triptans in Migraine without Aura. J Clin Pharmacol 59:288–294
Ray JC, Dalic L, Baker J, Cheng S, Hutton EJ, Matharu M (2024) Twelve-month efficacy of CGRP monoclonal antibodies and predictive value of short-term response: results of an Australian multicentre study. BMJ Neurol Open 6:e000547
Acknowledgements
The authors acknowledge the Center for Precision Medicine and Genomics at Tri-Service General Hospital, National Defense Medical Center for their assistance with statistical analysis.
Funding
This work was partially supported by grants from the Tri-Service General Hospital, Taiwan (grant numbers TSGH-D-113108, TSGH-D-113092, and TSGH-D112097) and Academia Sinica (grant numbers AS-40-05-GMM and AS-GC-110-MD02).
Author information
Authors and Affiliations
Contributions
Yu-Chin An and Fu-Chi Yang conceived and designed the study. Yu-Chin An, Kuo-Sheng Hung, and Fu-Chi Yang curated the data and performed the statistical analyses. Yu-Chin An, Kuo-Sheng Hung, and Fu-Chi Yang wrote the first draft of this manuscript. Po-Kuan Yeh, Chih-Sung Liang, Guan-Yu Lin, Chia-Lin Tsai, Sy-Jou Chen, Yu-Kai Lin, Yu-Chin An, Chia-Kuang Tsai, Kuo-Sheng Hung, and Fu-Chi Yang conducted the research and assisted with methodology development. Fu-Chi Yang supervised the planning and execution of the study. All authors contributed to manuscript revision and have read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare no competing interests. The study protocol was approved by the TSGH Institutional Review Board (TSGHIRB: 2-105-05-038). All individuals provided signed, informed consents before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
An, YC., Hung, KS., Liang, CS. et al. Genetic variants associated with response to anti-CGRP monoclonal antibody therapy in a chronic migraine Han Chinese population. J Headache Pain 25, 149 (2024). https://doi.org/10.1186/s10194-024-01850-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-024-01850-y