Abstract
Objective
The present study aimed to compare sex differences in the clinical manifestations related to dependence behaviors in medication-overuse headache (MOH).
Methods
Consecutive patients with newly diagnosed chronic migraine (CM) with and without MOH based on the Third Edition of International Classification of Headache Disorders (ICHD-3) were enrolled prospectively from the headache clinic of a tertiary medical center. Demographics and clinical profiles were collected by using a questionnaire, which included current use of tobacco, alcohol, and caffeinated beverages, the Leeds Dependence Questionnaire (LDQ), the Severity of Dependence Scale (SDS), the Headache Impact Test-6 (HIT-6), and the Pittsburgh Sleep Quality Index (PSQI).
Results
In total, 1419 CM patients (1135F/284 M, mean age 41.7 ± 13.9 years) were recruited, including 799 with MOH (640F/159 M, mean age 42.5 ± 13.2 years) (56.3%). Smoking was associated with an increased risk for MOH in men (odds ratio [OR] = 3.60 [95% confidence interval = 1.73–7.50], p = 0.001), but not in women (OR = 1.34 [0.88–2.04], p = 0.171) (p = 0.021 for interaction). Hypnotic use ≥ 3 days/week was a risk factor for MOH (OR = 2.55 [95% confidence interval = 2.00–3.24], p < 0.001), regardless of sex. By using receiver operating characteristics (ROC) curves, the cutoff scores of the LDQ for MOH were determined at 7 for women and 6 for men, and those for the SDS were 5 and 4, respectively (area under curve all ≥ 0.83). Among patients with MOH, the male sex was associated with a shorter latency between migraine onset and CM onset (12.9 ± 11.1 vs. 15.4 ± 11.5 years, p = 0.008), despite less average headache intensity (6.7 ± 1.9 vs. 7.2 ± 1.9, p = 0.005), functional impacts (HIT-6: 63.4 ± 8.3 vs. 65.1 ± 8.0, p = 0.009), and sleep disturbances (PSQI: 10.9 ± 4.4 vs. 12.2 ± 4.3, p = 0.001).
Conclusions
The current study identified an association between smoking and MOH in men, as well as sex-specific cutoffs of the LDQ and the SDS, for MOH. MOH was characterized by a shorter latency between migraine onset and CM onset in men and a more severe phenotype in women. Sex should be considered as an important factor in the evaluation of MOH.
Similar content being viewed by others
Background
Medication-overuse headache (MOH) is one of the leading causes of disease-related disability among all neurological disorders [1]. The estimated prevalence of MOH is about 1% in the general population [2,3,4,5], and it is associated with tremendous socioeconomic impacts [1, 5]. Timely diagnosis and management are indispensable in reducing the disease burden of MOH. In particular, it was found in the Chronic Migraine (CM) Epidemiology and Outcomes (CaMEO) study that medication overuse (MO) remained an important issue in about three fourths of CM patients, despite having had medical consultation, accurate diagnosis, and minimally appropriate medical treatment [6]. Therefore, MOH deserves more attention from the general public and medical professionals.
There is a growing interest in sex-related differences in medicine, especially in epidemiology, pathophysiology, clinical manifestations, and treatment response [7]. In clinical neurology and psychiatry, such a topic is gaining attention in headache disorders and substance use disorders (SUDs) [8,9,10]. Similar to CM, MOH is more common in women than in men [11,12,13]. However, there is little information about sex differences in the clinical manifestations of MOH. In particular, behaviors of substance dependence were reported to be present in up to two thirds of MOH patients [14,15,16,17]. In addition to acute medications, use of tobacco and other psychoactive substances is more common in patients with MOH than in those without [18, 19]. These findings are suggestive of shared pathophysiology with SUDs. Recently, there were reports pointing out the roles of sex-specific mechanisms in reward and addiction [20]. Whether the associations between MOH and clinical manifestations related to dependence behaviors could also be different in women and men is yet to be clarified. Such sex differences could have important clinical implications, and need to be elucidated.
The Leeds Dependence Questionnaire (LDQ) and the Severity of Dependence Scale (SDS) are two neuropsychological instruments commonly used in the clinical studies of dependence behaviors in SUDs [21, 22] and in MOH [23,24,25,26]. It was demonstrated that the LDQ and the SDS were useful to detect the presence of medication overuse (MO) or MOH [23,24,25,26], and the scores were correlated with the outcomes [17, 27, 28]. However, in many of the studies, comparisons were made between chronic daily headache (CDH) or CM patients with MOH and those with episodic migraine (EM), episodic cluster headache, or episodic tension-type headache (TTH) or even healthy controls. Besides, some of data were from population-based studies, namely the Akershus study [25, 26, 28]. The comparative performance of these two instruments in the detection of MOH among CM patients in the clinical settings is uncertain. More importantly, whether there could be sex differences in the diagnostic utilities of these two instruments for the detection of MOH among CM patients remains to be determined.
The aims of the present study were (1) to determine sex differences in the association between MOH and the current use of tobacco, alcohol, caffeine, or hypnotics, (2) to compare the diagnostic utilities of the LDQ and the SDS in the detection of MOH between women and men with CM, and (3) to evaluate between-sex differences in the clinical presentations of MOH.
Methods
Patients
In this prospective study, patients with newly diagnosed CM with and without a concomitant diagnosis of MOH between September 2018 and November 2022 were enrolled consecutively. Patients were recruited at their first visit to the Headache Clinic of Taipei Veterans General Hospital, a tertiary medical center in the capital city of Taiwan. The Taiwan National Health Insurance (NHI) covers > 99% of the population of our country. Although a referral mechanism is included in the Taiwan NHI, it is not mandatory. Most of the medical expenses are reimbursed, and copayment associated with direction consultations with specialists, even in tertiary medical centers, without referral is typically minimal [29].
Headache diagnoses were made by headache specialists according to the diagnostic criteria of the Third Edition of the International Classification of Headache Disorders (ICHD) (ICHD-3) [30]. The inclusion criteria were (a) willingness to participate in the study, (b) age between 20 and 65 years, and (c) fulfillment of the ICHD-3 criteria for migraine. The exclusion criteria included (a) episodic migraine, (b) coexistence of an acute headache disorder (within one month of headache onset), (c) coexistence of a secondary headache disorder, and (d) difficulties completing the history taking or the questionnaire-based interview. The study protocols were approved by the Institutional Review Board of Taipei Veterans General Hospital (TVGH IRB No. 2018–07-020BC, and 2019–07-002CC). All of the patients gave written informed consent before entering the study.
Questionnaire-based interviews
Demographics, clinical profiles, headache characteristics, and behaviors of dependence were collected by using a specifically designed questionnaire. The current use of tobacco, alcohol, and caffeinated beverages (coffee, tea, Coke, etc.) was queried specifically, and the responses were treated as binary variables. The 6-item Headache Impact Test (HIT-6) was used to measure the negative impact on daily activities resulting from headache [31]. The Hospital Anxiety and Depression Scale (HADS) was used to screen for psychological disturbances, and included subscales for anxiety (HADS-A) and depression (HADS-D) [32]. The quality of sleep was assessed by using the Pittsburg Sleep Quality Index (PSQI) [33], which included a question for the status of hypnotic use (none, < 1 day/week, 1–2 days/week, ≥ 3 days/week). Frequent hypnotic use was defined as hypnotic use ≥ 3 days/week, the frequency of which was close to the definition of MOH on the ICHD-3 [30]. The severity of dependence behaviors was rated by using modified versions of the LDQ and the SDS for the use in headache disorders [21,22,23, 25]. The scores of these instruments were verified by headache specialists at face-to-face interviews.
The modified version of LDQ consists of ten questions [23], each of which is to be rated on a scale of 0 to 3 (0 = never, 1 = sometimes, 2 = often, 3 = nearly always). The total score ranges from 0 to 30. The questions are as follows: 1. Do you find yourself thinking about when you will next be able to take analgesics? 2. Is taking analgesics more important than anything else you might do during the day? 3. Do you feel your need for analgesics is too strong to control? 4. Do you plan your days around taking analgesics? 5. Do you take analgesic in a particular way in order to increase the effect it gives you? 6. Do you take analgesics morning, afternoon and evening? 7. Do you feel you have to carry on taking analgesics once you have started? 8. Is getting the effect you want more important than the particular analgesic you use? 9. Do you want to take more analgesics when the effect starts to wear off? 10. Do you find it difficult to cope with life without analgesics?
There are five questions in the modified version of SDS [25], and each item is graded and scored between 0 and 3. The score “0” denotes never/almost never for questions 1–4 and not difficult for question 5, and the score “3” indicates always/nearly always for questions 1–4 and impossible for question 5. The range of the total score lies between 0 and 15. The questions are as follows: 1. Do you think your use of your headache medication is out of control? 2. Does the prospect of missing a dose make you anxious or worried? 3. Do you worry about your use of your headache medication(s)? 4. Do you wish you could stop? 5. How difficult do you find it to stop or go without your headache medication?
Statistical analysis
Continuous variables were compared by using the Student’s t test between groups, or the Mann–Whitney U test if the null hypothesis of normality was rejected. Categorical variables were compared by using the chi-square test. The optimum cutoff scores of the LDS and SDS for detecting the presence of MOH were determined by using receiver operating characteristics (ROC) curves, in conjunction with Youden’s J statistics. The discriminative performances of these two instruments were evaluated by the areas under the ROC curves (AUC). In general, an AUC of 0.7 to 0.8 indicates acceptable, 0.8 to 0.9 excellent, and > 0.9 outstanding accuracy of a diagnostic test [34]. Logistic regression modeling (enter method) was carried out to estimate the odds ratios (ORs) and the 95% confidence intervals (CIs) for having MOH in relation to the status of smoking, before and after taking potential confounders into consideration. Statistical analysis was carried out by using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as a two-sided p of < 0.05.
Results
Study participants
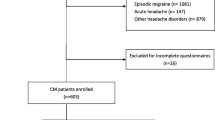
During the study period, 4233 consecutive migraine patients were screened at their first visit, and 2814 patients were excluded for being diagnosed as episodic migraine (n = 2709), coexistence of other headache disorders (n = 7) or acute headache (n = 5), or incomplete questionnaires (n = 93) (Fig. 1). In total, 1419 CM patients (1135F/284 M, mean age 41.7 ± 13.9 years) were included in the analysis, 799 of which had a coexisting diagnosis of MOH (56.3%) (640F/159 M, mean age 42.5 ± 13.2 years) (Table 1). Among patients with MOH (n = 799), acute medications being overused were multiple medications in 439 (54.9%), acetaminophen in 167 (20.9%), combination analgesics in 81 (10.1%), nonsteroidals in 25 (3.1%), cold syrups in 19 (2.4%), triptans in 13 (1.6%), tramadol in 11 (1.4%), ergots in 13 (1.6%), others in 10 (1.3%), and unknown in 21 (2.6%). Among patients overusing multiple medications (n = 439), 253 (57.6%), 132 (30.1%), and 54 (12.3%) used two, three, and four or more medications, respectively, and the mostly commonly used combinations include acetaminophen plus combination analgesics (n = 104, 23.7%), acetaminophen plus NSAIDs (n = 39, 8.9%), acetaminophen plus ergots (n = 21, 4.8%), etc.
Patient with MOH were older than those without (mean age: 42.5 ± 13.2 vs. 40.6 ± 14.6 years, p = 0.008) (Table 1), and had an earlier onset of migraine (onset age 20.8 ± 10.0 vs. 23.1 ± 11.3 years, p < 0.001), a longer duration of CM (92.9 ± 110.4 vs. 59.7 ± 99.5 months, p < 0.001), greater average headache intensity (7.1 ± 1.9 vs. 6.1 ± 1.9, p < 0.001), a higher frequency of acute medication use (19.7 ± 7.8 vs. 4.0 ± 5.5 days/month, p < 0.001), a greater headache impact (HIT-6: 64.8 ± 8.1 vs. 62.5 ± 7.0, p < 0.001), more symptoms of depression (HADS-D: 8.1 ± 4.6 vs. 7.4 ± 4.4, p = 0.002), and poorer sleep quality (PSQI: 12.0 ± 4.3 vs. 11.0 ± 4.1, p < 0.001). Besides, patients with MOH had higher total scores on the LDQ (12.8 ± 7.8 vs. 3.9 ± 5.0, p < 0.001) and the SDS (6.9 ± 3.9 vs. 2.3 ± 2.8, p < 0.001) than those without (Table 1).
Association between smoking and MOH, especially in men
Tobacco use was more common in patients with MOH (17.2% vs. 9.1%, p < 0.001) than in those without, but the proportions of patients reporting current use of alcohol (30.6% vs. 33.7%, p = 0.220) or caffeinated beverages (65.1% vs. 64.2%, p = 0.519) were similar. In univariate analysis, smoking was associated with increased odds of MOH (OR = 2.08 [95% CI = 1.49–2.89], p < 0.001). There was a significant sex-by-smoking interaction (OR = 2.33 [1.08–5.03], p = 0.031), and the association was stronger in men (OR = 3.87 [1.99–7.54], p < 0.001) than in women (OR = 1.66 [1.13–2.44], p = 0.010). After taking age, the number of monthly headache days, CM duration (years), and scores on HADS-A, HADS-D, and PSQI into consideration, there was still a significantly sex-by-smoking interaction (OR = 2.63 [1.16–5.98], p = 0.021), and the association between smoking and MOH remained significant in men (OR = 3.60 [1.73–7.50], p = 0.001), but not in women (OR = 1.34 [0.88–2.04], p = 0.171) (Table 2). The findings were similar when either LDQ or SDS scores were further included in the model (data not shown). However, current use of alcohol or caffeinated beverages was not associated with the presence of MOH (data not shown).
On the other hand, frequent hypnotic use, i.e. ≥ 3 days/week, was more common in patients with MOH (40.5% vs. 21.0%, p < 0.001) than in those without, and was associated with increased odds of having MOH (OR = 2.55 [95% confidence interval = 2.00–3.24], p < 0.001). However, there was no significant sex difference (OR = 1.31 [0.68–2.52], p = 0.426 for sex-by-frequent-hypnotic-use interaction). The findings were similar after controlling for potential confounders (data not shown).
Diagnostic utilities of LDQ and SDS for MOH in women and men
In the entire study population, the cutoff score of the LDQ for a diagnosis of MOH was determined at 7, with a sensitivity of 75.7% and a specificity of 78.4% (AUC = 0.85), and the cutoff score of the SDS was determined at 5 (sensitivity = 73.1%, specificity = 79.5%, AUC = 0.84) (Fig. 2A).
When women and men were analyzed separately, the cutoff scores of the LDQ for MOH were determined at 7 for women (sensitivity = 75.9%, specificity = 78.0%, AUC = 0.84) and 6 for men (sensitivity = 80.5%, specificity = 79.2%, AUC = 0.87) (Fig. 2B-C), and the cutoff scores for the SDS were 5 for women (sensitivity = 74.0%, specificity = 79.6%, AUC = 0.84) and 4 for men (sensitivity = 76.7%, specificity = 73.6%, AUC = 0.83).
Acute medication use and clinical correlations in patients with MOH
When individual acute medications were analyzed separately, the most commonly used acute medications were acetaminophen (65.8%), NSAIDs (58.2%), ergots (19.6%), and triptans (13.1%). NSAID use was more frequent in women than in men (60.3% vs. 49.7%, p = 0.015), whereas men were more likely to use cold syrups (13.2% vs. 7.8%, p = 0.032) and tramadol (11.3% vs. 6.1%, p = 0.022) (Table 3). Triptan users scored higher on the LDQ (14.8 ± 7.7 vs. 12.5 ± 7.8, p = 0.004) and the SDS (7.9 ± 3.8 vs. 6.8 ± 3.9, p = 0.008) when compared with non-users. The findings were similar for ergot users (LDQ: 14.5 ± 7.8 vs. 12.4 ± 7.7, p = 0.003; SDS: 8.1 ± 4.0 vs. 6.7 ± 3.8, p < 0.001). On the other hand, acetaminophen use was associated with lower SDS scores (6.7 ± 3.9 vs. 7.4 ± 3.9, p = 0.021), although the LDQ scores were comparable (12.8 ± 7.8 vs. 12.9 ± 7.8, p = 0.777). There was no difference for other acute medication, and the trends were generally consistent between the sexes (data not shown).
Sex differences in clinical manifestations of patients with MOH
When compared with men, women with MOH were older (mean age: 43.0 ± 13.1 vs. 40.5 ± 13.4 years, p = 0.030), had greater average headache intensity (7.2 ± 1.9 vs. 6.7 ± 1.9, p = 0.005) and headache impact (HIT-6: 65.1 ± 8.0 vs. 63.4 ± 8.3, p = 0.009), and poorer sleep (PSQI: 12.2 ± 4.3 vs. 10.9 ± 4.4, p = 0.001), and were more likely to have frequent hypnotic use (i. e. ≥ 3 days/week) (42.2% vs. 33.5%, p = 0.048) (Table 3). Besides, women were more likely to have nausea (89.5% vs. 81.1%, p = 0.004) and vomiting (49.4% vs. 37.7%, p = 0.008) as headache-associated symptoms. On the other hand, the latency between migraine onset and CM onset was shorter in men than in women (12.9 ± 11.1 vs. 15.4 ± 11.5 years, p = 0.008). The proportions of patients reporting tobacco (31.0% vs. 13.7%, p < 0.001) or alcohol (44.7% vs. 27.1%, p < 0.001) use were higher in men than in women with MOH. However, other clinical headache features and associated symptoms were similar between the sexes.
In exploratory analysis based on sex, female MOH patients with current tobacco use had more frequent acute medication use (22.0 ± 7.3 vs. 19.3 ± 7.8 days/month, p = 0.002) and shorter intervals between migraine onset and CM onset (12.7 ± 9.4 vs. 15.6 ± 11.7 years, p = 0.019) than those without, despite similar age at migraine onset (20.3 ± 9.3 vs. 20.7 ± 10.1 years, p = 0.713) and headache frequencies (24.5 ± 6.2 vs. 23.6 ± 6.4 days/month, p = 0.235). On the other hand, the frequencies of acute medication use (19.0 ± 7.9 vs. 20.0 ± 7.6 days/month, p = 0.433) and intervals between migraine onset and CM onset (12.7 ± 10.6 vs. 12.8 ± 11.4 years, p = 0.964) were comparable between men with MOH who smoked and who did not, and so were the ages at migraine onset and headache frequencies (data not shown).
Discussion
In the current study, it was found that smoking was independently associated with MOH in men, but not in women, and frequent hypnotic use was associated with MOH, regardless of sex. Besides, the LDQ and the SDS were of excellent diagnostic performances for the diagnosis of MOH in patients with CM of both sexes, although the cutoff scores for both of the instruments were one point lower in men than in women. In addition, MOH in the current cohort was characterized by a more severe phenotype in women, and a shorter latency between migraine onset and CM onset in men. Sex should be considered as an important factor in the initial evaluation of patients with suspected MOH.
Important strengths of the present study included sample size, quality of the data, and study design. The current study recruited more than 1400 consecutive CM patients. Therefore, selection bias could be reduced, and more accurate estimates could be derived. In particular, the number of male patients in the present study was larger than those in the majority of prior studies. Besides, the data were of high quality and reliability. The diagnoses of CM and MOH were made by headache specialists according to the ICHD-3 criteria. The data were collected systematically by using a specifically designed questionnaire. In addition, the clinical utilities of the LDQ and the SDS in CM patients with and without MOH were compared directly. In addition, dependence behaviors and related manifestations were compared between the sexes. Therefore, the present study could provide more comprehensive and complete information that is more relevant to CM patients evaluated in the clinical settings.
It was found that smoking was associated with MOH, and the association was stronger in men. An association between smoking and MOH, as well as SUDs, was reported in the literature [19, 35,36,37], although whether there could be sex differences has rarely been discussed upon. Since smoking shares some clinical features with SUDs, it is possible that tobacco use could reflect the presence of disturbances in the reward circuit such that these patients were at risk of developing MOH. On the other hand, frequent hypnotic use was also associated with MOH, which was in keeping with the association between MOH and tranquilizers reported in the literature [19, 38]. However, there was no sex difference. Interpretations for the associations with alcohol and caffeine use could be more complicated since these agents could have direct impacts on migraine attacks [39]. In addition to the mechanisms related to reward circuit, it is also possible that the identified association between MOH and smoking and hypnotic use could also be attributed to certain shared factors, such as socioeconomic status, stress levels, lifestyles, etc. Further studies are needed to clarify the roles of these potential confounders. Interestingly, smoking cessation could have a positive effect on the treatment outcome of SUDs [40]. Whether smoking cessation would reduce the risk or improve the prognosis of MOH, especially in men, deserves further exploration.
The current MOH cohort was characterized by a low rate of triptan use, and there was an association between overuse of triptans or ergots and more severe dependence behaviors. The rate of triptan use or overuse in our MOH patients was lower than estimates from some studies [41,42,43], although the trend was consistent with prior reports from Taiwan, which could be attributed to the costs of tritpans and the restrictions on their use, e.g. ≤ 400 mg/month for sumatriptan tablets, by the reimbursement regulations of the Taiwan NHI [29, 44]. In the present study, triptan or ergot users scored higher on the LDQ and the SDS. In fact, it takes shorter latencies and lower doses for patients overusing triptans or ergots to develop MOH than those with simple analgesic overuse [41]. These findings could be suggestive of an association between overuse of these two classes of acute medications and a greater tendency to develop MOH. Alternatively, it is also possible that patients with different headache severities or personality traits would preferably overuse certain categories of acute medications [45, 46]. However, interpretations could be cautious since the categories of triptan or ergot overuse included patients using these agents alone or in combination with other acute medications. More studies are needed to provide more insight into the underlying pathophysiology.
In the current study, the LDQ and the SDS were of comparable accuracies in the detection of MOH in CM patients of both sexes. It was reported that the severity of dependence behaviors, as measured by the LDQ, in CDH patients with MO was greater than those with episodic primary headache disorders, and was similar to that in SUDs involving alcohol or illicit drugs [23]. Besides, it was demonstrated that an SDS score of ≥ 5 was correlated with the presence of MO in the general population [25], as well as behaviors of substance dependence in the clinical settings [17], and the cutoff score identified in our study was the same. In MOH patients who received detoxification, responders had greater decrease in the LDQ scores [27]. Besides, the SDS scores at baseline were predictive of the outcome in MOH associated with primary headache disorders [28], and the SDS could decrease following withdrawal of acute medications [47]. In addition to preventive medications, behavioral therapy targeting at dependence behaviors could also be an important treatment option to improve the treatment outcome [14]. It was demonstrated that brief intervention, which consisted of feedback based on the initial scores of SDS and consultations to reduce the use of acute medication, was an effective for MOH patients [48]. It is possible that both the LDQ and the SDS could have important roles not only as screening instruments, but also in the treatment and prognostication.
In the present study, it was found that cutoff scores for the diagnosis of MOH were lower in men than in women for both of the instruments, and there were differences in clinical manifestations of MOH and medication use between the sexes. Actually, it was demonstrated in a population-based study that SDS ≥ 5 for women and ≥ 4 for men could help detect MO in a group of patients with primary chronic headache disorders, of whom 95% had chronic TTH [25], and the cutoffs identified in our study were exactly the same. In fact, there have been some reports on sex-specific cutoffs in the diagnosis a variety of diseases, such as right bundle branch block, myocardial infarction, venous thromboembolism, etc. [49,50,51,52], which indicate that an uniform diagnostic cutoff score may not always be the best strategy. On the other hand, it was found that MOH in women was associated with more severe clinical manifestations, which is consistent with the trend in migraine and CM [9, 12, 53]. Besides, MOH in men was characterized by a shorter interval between migraine onset and CM onset. In fact, among patients with EM at baseline, men were more likely to have CM onset at 6, 9, and 12 months in the CaMEO study [54]. This might indicate that migraine chronification could take a shorter period of time to develop in men. Since there is a female preponderance in both CM and MOH [11,12,13], data in male patients could be under-presented in the literature. Clinical decisions for men with MOH based on available evidence could be biased. Further studies are needed to provide more insights on sex-specific differences on such an issue.
There are some limitations. First of all, the patients were recruited from the headache clinic of a tertiary medical center, and only patients who were 20–65 years old were included in the study. There could be concerns about generalizability. However, there is no strict regulation on the process of referral in the healthcare system of our country, and the majority of patients came in directly without referral. Therefore, patients involved in the present study could reflect those in the general population to a certain extent. Second, both the LDQ and the SDS were administered by the patients themselves, and there could be concerns about reliability. However, the responses in these instruments were verified by headache specialists at face-to-face interviews. Besides, the self-administered version of SDS has been validated against headache specialist interviews by telephone [55]. Third, dependence behaviors in the current study were defined based on the scores on neuropsychological instruments, rather than formal psychiatric evaluation. In fact, although MOH shares many clinical and neuroimaging features with SUDs, certain important elements of SUD are not seen in patients with MOH, such as craving, drug-associated cues, impulsivity, and compulsive behaviors [56, 57]. Besides, controversies remain as to whether overuse of acute medications could just reflect the severity of the underlying headache disorders [45], since most of the commonly used acute medications are generally considered to be of low abuse potential [58, 59]. Therefore, the findings should be interpreted with caution. Fourth, the use of tobacco, alcohol, and caffeinated beverages was defined by dichotomous variables, and whether the amount or frequency of their use to have an impact is yet to be determined. Finally, as a cross-sectional study, only associations, rather than causal relationships, could be identified, and the findings should be interpreted with caution.
In conclusion, the present study demonstrated differential risks of MOH associated with tobacco use between men and women and identified sex-specific cutoff scores of the LDQ and the SDS for MOH among patients with CM. Besides, compared with men, women with MOH seemed to have longer latencies of migraine chronification, except for those who smoke. Sex should be taken into account in the evaluation of MOH.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to participant confidentiality.
Abbreviations
- AUC:
-
Area under curve
- CI:
-
Confidence interval
- CaMEO:
-
Chronic Migraine Epidemiology and Outcomes
- CDH:
-
Chronic daily headache
- CM:
-
Chronic migraine
- HADS:
-
Hospital Anxiety and Depression Scale
- HADS-A:
-
Anxiety subscale of Hospital Anxiety and Depression Scale
- HADS-D:
-
Depression subscale of Hospital Anxiety and Depression Scale
- HIT-6:
-
Headache Impact Test-6
- ICHD-3:
-
Third Edition of the International Classification of Headache Disorders
- LDQ:
-
Leeds Dependence Questionnaire
- MOH:
-
Medication-overuse headache
- OR:
-
Odds ratio
- ROC:
-
Receiver operating curve
- SDS:
-
Severity of Dependence Scale
- SUD:
-
Substance use disorder
- TTH:
-
Tension-type headache
References
Group GBDNDC (2017) Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16(11):877–97
Wang SJ, Fuh JL, Lu SR, Liu CY, Hsu LC, Wang PN et al (2000) Chronic daily headache in Chinese elderly: prevalence, risk factors, and biannual follow-up. Neurology 54(2):314–319
Lu SR, Fuh JL, Chen WT, Juang KD, Wang SJ (2001) Chronic daily headache in Taipei, Taiwan: prevalence, follow-up and outcome predictors. Cephalalgia 21(10):980–986
Wang SJ, Fuh JL, Lu SR, Juang KD (2006) Chronic daily headache in adolescents: prevalence, impact, and medication overuse. Neurology 66(2):193–197
Chen PK, Wang SJ (2019) Medication overuse and medication overuse headache: risk factors, comorbidities, associated burdens and nonpharmacologic and pharmacologic treatment approaches. Curr Pain Headache Rep 23(8):60
Buse DC, Armand CE, Charleston LT, Reed ML, Fanning KM, Adams AM et al (2021) Barriers to care in episodic and chronic migraine: results from the chronic migraine epidemiology and outcomes study. Headache. 61(4):628–41
Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL et al (2020) Sex and gender: modifiers of health, disease, and medicine. Lancet 396(10250):565–582
The LN (2019) A spotlight on sex differences in neurological disorders. Lancet Neurol 18(4):319
Vetvik KG, MacGregor EA (2017) Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 16(1):76–87
McHugh RK, Votaw VR, Sugarman DE, Greenfield SF (2018) Sex and gender differences in substance use disorders. Clin Psychol Rev 66:12–23
Ashina S, Terwindt GM, Steiner TJ, Lee MJ, Porreca F, Tassorelli C et al (2023) Medication overuse headache. Nat Rev Dis Primers 9(1):5
Tsai CK, Tsai CL, Lin GY, Yang FC, Wang SJ (2022) Sex differences in chronic migraine: focusing on clinical features, pathophysiology, and treatments. Curr Pain Headache Rep 26(5):347–355
Buse DC, Manack AN, Fanning KM, Serrano D, Reed ML, Turkel CC et al (2012) Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache 52(10):1456–1470
Fuh JL, Wang SJ, Lu SR, Juang KD (2005) Does medication overuse headache represent a behavior of dependence? Pain 119(1–3):49–55
Radat F, Creac’h C, Guegan-Massardier E, Mick G, Guy N, Fabre N et al (2008) Behavioral dependence in patients with medication overuse headache: a cross-sectional study in consulting patients using the DSM-IV criteria. Headache 48(7):1026–1036
Bottiroli S, Galli F, Ballante E, Pazzi S, Sances G, Guaschino E et al (2022) Validity of the severity of dependence scale for detecting dependence behaviours in chronic migraine with medication overuse. Cephalalgia 42(3):209–217
Lundqvist C, Gossop M, Russell MB, Straand J, Kristoffersen ES (2019) Severity of analgesic dependence and medication-overuse headache. J Addict Med 13(5):346–353
Radat F, Creac’h C, Swendsen JD, Lafittau M, Irachabal S, Dousset V et al (2005) Psychiatric comorbidity in the evolution from migraine to medication overuse headache. Cephalalgia 25(7):519–522
Hagen K, Linde M, Steiner TJ, Stovner LJ, Zwart JA (2012) Risk factors for medication-overuse headache: an 11-year follow-up study The Nord-Trondelag Health Studies. Pain 153(1):56–61
Becker JB, Chartoff E (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44(1):166–183
Raistrick D, Bradshaw J, Tober G, Weiner J, Allison J, Healey C (1994) Development of the Leeds Dependence Questionnaire (LDQ): a questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction 89(5):563–572
Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W et al (1995) The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 90(5):607–614
Ferrari A, Cicero AF, Bertolini A, Leone S, Pasciullo G, Sternieri E (2006) Need for analgesics/drugs of abuse: a comparison between headache patients and addicts by the Leeds Dependence Questionnaire (LDQ). Cephalalgia 26(2):187–193
Wang YF, Tzeng YS, Yu CC, Ling YH, Chen SP, Lai KL et al (2023) Clinical utility of leeds dependence questionnaire in medication-overuse headache. Diagnostics (Basel) 13(3):472
Grande RB, Aaseth K, Saltyte Benth J, Gulbrandsen P, Russell MB, Lundqvist C (2009) The severity of dependence scale detects people with medication overuse: the Akershus study of chronic headache. J Neurol Neurosurg Psychiatry 80(7):784–789
Lundqvist C, Benth JS, Grande RB, Aaseth K, Russell MB (2011) An adapted Severity of Dependence Scale is valid for the detection of medication overuse: the Akershus study of chronic headache. Eur J Neurol 18(3):512–518
Corbelli I, Caproni S, Eusebi P, Sarchielli P (2012) Drug-dependence behaviour and outcome of medication-overuse headache after treatment. J Headache Pain 13(8):653–660
Lundqvist C, Grande RB, Aaseth K, Russell MB (2012) Dependence scores predict prognosis of medication overuse headache: a prospective cohort from the Akershus study of chronic headache. Pain 153(3):682–686
Wang YF, Wang SJ, Huang YH, Chen YT, Yen YC, Shia BC et al (2023) Treatment pattern and health care resource utilization for Taiwanese patients with migraine: a population-based study. Front Neurol 14:1222912
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. https://doi.org/10.1177/0333102417738202.
Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A et al (2003) A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 12(8):963–974
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
Mandrekar JN (2010) Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 5(9):1315–1316
Schwedt TJ, Alam A, Reed ML, Fanning KM, Munjal S, Buse DC et al (2018) Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study. J Headache Pain 19(1):38
Sances G, Ghiotto N, Galli F, Guaschino E, Rezzani C, Guidetti V et al (2010) Risk factors in medication-overuse headache: a 1-year follow-up study (care II protocol). Cephalalgia 30(3):329–336
Lai S, Lai H, Page JB, McCoy CB (2000) The association between cigarette smoking and drug abuse in the United States. J Addict Dis 19(4):11–24
Viana M, Bottiroli S, Sances G, Ghiotto N, Allena M, Guaschino E et al (2018) Factors associated to chronic migraine with medication overuse: A cross-sectional study. Cephalalgia 38(14):2045–2057
Zaeem Z, Zhou L, Dilli E (2016) Headaches: a Review of the Role of Dietary Factors. Curr Neurol Neurosci Rep 16(11):101
McKelvey K, Thrul J, Ramo D (2017) Impact of quitting smoking and smoking cessation treatment on substance use outcomes: An updated and narrative review. Addict Behav 65:161–170
Limmroth V, Katsarava Z, Fritsche G, Przywara S, Diener HC (2002) Features of medication overuse headache following overuse of different acute headache drugs. Neurology 59(7):1011–1014
Zeeberg P, Olesen J, Jensen R (2006) Probable medication-overuse headache: the effect of a 2-month drug-free period. Neurology 66(12):1894–1898
de Rijk P, Resseguier N, Donnet A (2018) Headache characteristics and clinical features of elderly migraine patients. Headache 58(4):525–533
Wang SJ, Fuh JL (2008) Medication overuse headache in Taiwan. Cephalalgia 28(11):1240–1242
Bigal ME, Lipton RB (2008) Excessive acute migraine medication use and migraine progression. Neurology 71(22):1821–1828
Mose LS, Pedersen SS, Jensen RH, Gram B (2019) Personality traits in migraine and medication-overuse headache: A comparative study. Acta Neurol Scand 140(2):116–122
Rouw C, Munksgaard SB, Engelstoft IMS, Nielsen M, Westergaard ML, Jensen RH et al (2021) Dependence-like behaviour in patients treated for medication overuse headache: A prospective open-label randomized controlled trial. Eur J Pain 25(4):852–861
Kristoffersen ES, Straand J, Vetvik KG, Benth JS, Russell MB, Lundqvist C (2015) Brief intervention for medication-overuse headache in primary care. The BIMOH study: a double-blind pragmatic cluster randomised parallel controlled trial. J Neurol Neurosurg Psychiatry. 86(5):505–12
Strauss DG, Selvester RH, Wagner GS (2011) Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol 107(6):927–934
Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA et al (2014) Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 63(14):1441–1448
Peacock WF, Baumann BM, Rivers EJ, Davis TE, Handy B, Jones CW et al (2021) Using sex-specific cutoffs for high-sensitivity cardiac troponin T to diagnose acute myocardial infarction. Acad Emerg Med 28(4):463–466
Reagh JJ, Zheng H, Stolz U, Parry BA, Chang AM, House SL et al (2021) Sex-related differences in D-dimer levels for venous thromboembolism screening. Acad Emerg Med 28(8):873–881
Wang SJ, Fuh JL, Juang KD, Lu SR (2005) Rising prevalence of migraine in Taiwanese adolescents aged 13–15 years. Cephalalgia 25(6):433–438
Scher AI, Wang SJ, Katsarava Z, Buse DC, Fanning KM, Adams AM et al (2019) Epidemiology of migraine in men: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Cephalalgia 39(2):296–305
Kristoffersen ES, Benth JS, Straand J, Russell MB, Lundqvist C (2019) Validity of self-reported assessment of Severity of Dependence Scale in Medication-Overuse Headache. Scand J Pain 19(4):837–841
Takahashi TT, Ornello R, Quatrosi G, Torrente A, Albanese M, Vigneri S et al (2021) Medication overuse and drug addiction: a narrative review from addiction perspective. J Headache Pain 22(1):32
Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A et al (2013) DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 170(8):834–851
Ferrari A, Leone S, Tacchi R, Ferri C, Gallesi D, Giuggioli D et al (2009) The link between pain patient and analgesic medication is greater in migraine than in rheumatic disease patients. Cephalalgia 29(1):31–37
Abbott FV, Fraser MI (1998) Use and abuse of over-the-counter analgesic agents. J Psychiatry Neurosci 23(1):13–34
Acknowledgements
The authors would like to thank the patients for their help.
Funding
This study was funded in part by Taiwan National Science and Technology Council (formerly Taiwan Ministry of Science and Technology) [109–2314-B-075 -054 and 110–2314-B-075 -041 -MY3 (to Y.F.W.), and 104–2314-B-010–015-MY2, 106–2321-B-010–009, 107–2321-B-010–001, 108–2321-B-010–014 -MY2, 108–2321-B-010 001, 108–2314-B-010–023-MY3, and 110–2321-B-010–005 (to S.J.W.)]; and Taipei Veterans General Hospital [V108C-092, V109C-096, V110C-111, V111C-161, V112C-078, and V112D67-003-MY3-1 (to Y.F.W.)]; this work was also supported by the Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.F.W. and S.J.W. conceived and designed the study, and completed the first draft, tables and figures. Y.F.W, Y.H.L., S.P.C, K.L.L., W.T.C., and S.J.W. acquired the data. Y.F.W., Y.S.T., C.C.Y., W.T.C., and S.J.W. analyzed the data. Y.F.W, Y.S.T, C.C.Y., Y.H.L., S.P.C, K.L.L., W.T.C., and S.J.W. participated in the discussion and critical revision of the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocols were approved by the Institutional Review Board of Taipei Veterans General Hospital (VGH IRB No. 2018–07-020BC, and 2019–07-002CC).
Consent for publication
Not applicable.
Competing interests
Y.F.W. has received honoraria as a speaker from Taiwan branches of Allergan/AbbVie, Boehringer Ingelheim, Chugai, Eli Lilly, Novartis, Pfizer, Sanofi, UCB, and Viatris, Hava Bio-Pharma, Orient EuroPharma, and Teva. He has received research grants from the Taiwan National Science and Technology Council (formerly Taiwan Ministry of Science and Technology), and Taipei Veterans General Hospital. S.J.W. has served on the advisory boards of Daiichi-Sankyo, Eli Lilly and Novartis; has received honoraria as a moderator from Allergan/AbbVie, Pfizer, Eli Lilly, Biogen and Eisai and has been the PI in trials sponsored by Eli Lilly, Novartis, and Allergan/AbbVie. He has received research grants from the Taiwan National Science and Technology Council (formerly Taiwan Ministry of Science and Technology); Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Taiwan Ministry of Education; Taipei Veterans General Hospital; Taiwan Headache Society; and Taiwan branches of Eli Lilly, Novartis, and Pfizer. Y.S.T, C.C.Y., Y.H.L., S.P.C, K.L.L., and W.T.C. reported no relevant conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, YF., Tzeng, YS., Yu, CC. et al. Sex differences in the clinical manifestations related to dependence behaviors in medication-overuse headache. J Headache Pain 24, 145 (2023). https://doi.org/10.1186/s10194-023-01685-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-023-01685-z