Abstract
Background
Task-free imaging approaches using PET have shown the posterior hypothalamus to be specifically activated during but not outside cluster headache attacks. Evidence from task related functional imaging approaches however is scarce.
Methods
Twenty-one inactive cluster headache patients (episodic cluster headache out of bout), 16 active cluster headache patients (10 episodic cluster headache in bout, 6 chronic cluster headache) and 18 control participants underwent high resolution brainstem functional magnetic resonance imaging of trigeminal nociception using gaseous ammonia as a painful stimulus.
Results
Following trigeminonociceptive stimulation with ammonia there was a significantly stronger activation within the posterior hypothalamus in episodic cluster headache patients out of bout when compared to controls. When contrasting estimates of the pain contrast, active cluster headache patients where in between the two other groups but did not differ significantly from either.
Conclusion
The posterior hypothalamus might thus be hyperexcitable in cluster headache patients outside the bout while excitability to external nociceptive stimuli decreases during in bout periods, probably due to frequent hypothalamic activation and possible neurotransmitter exhaustion during cluster attacks.
Similar content being viewed by others
Introduction
In most Task-free imaging approaches like H2O-PET and restingstate fMRI, the posterior hypothalamus has been shown to be specifically activated during but not outside cluster headache attacks with activity levels normalising after pain relieve due to sumatriptan administration [1,2,3]. Furthermore, hypothalamic restingstate connectivity is altered in cluster headache patients as compared to healthy controls [4, 5] thus suggesting a crucial role of this brain area in the pathophysiology of cluster headache Evidence from Task-related functional imaging approaches however is scarce – to our knowledge there are to date no stimulation fMRI studies in cluster headache patients although investigating the trigeminal nociceptive functioning in active and inactive cluster headache might add important information to our current understanding of cluster headache pathophysiology. There is however some evidence of altered nociceptive and autonomic functioning in cluster headache: temporal nociceptive processing seems to be facilitated during the active cluster headache phases while markers of supraspinal pain control might be defective [6]. Accordingly, in cluster headache patients there is evidence for trigeminonociceptive facilitation on brainstem level [7]. Here we hypothesized that hypothalamic processing of trigeminal nociceptive stimuli might be altered in cluster headache patients as compared to healthy controls. We investigate active and non-active cluster headache patients using a well-established paradigm for functional magnetic resonance imaging of trigeminal nociception.
Methods
Participants
Cluster headache patients were recruited via the Headache Outpatient Department of the University Medical Center Eppendorf in Hamburg, Germany, and a local database between August 2014 and November 2016. Patients were divided into 2 groups: active cluster headache (including both episodic cluster headache patients in bout and chronic cluster headache patients) and inactive cluster headache (episodic cluster headache patients out of bout). Three patients participated both in the active state and in the inactive state. Control participants did not suffer from any severe neurological or internal illness and had no history of pain or headache diseases including migraine, cluster headache and frequent tension type headache. Written informed consent was obtained from all participants.
Experimental paradigm
Participants underwent one session of event-related fMRI using a previously established protocol of standardized nociceptive stimulation of the nasal mucosa using gaseous ammonia [8,9,10,11,12,13]. Additionally, 3 of the episodic cluster headache patients underwent a second identical session within bout period. Each session consisted of three parts, during which 4 different stimuli were presented in pseudorandomized order ensuring no two identical stimuli directly followed each other. Each stimulus was preceded by a reaction task and followed by a rating procedure during which intensity and unpleasantness of the respective stimulus had to be rated. The 4 stimuli were gaseous ammonia as an activator of trigeminonociceptive afferents within the nasal mucosa, rose odour as a purely olfactory stimulus, air puffs as a control condition and a rotating checkerboard as visual stimulus. The gaseous stimuli were presented via a Teflon tube within the participants’ nostril (in case of cluster headache patients on the site of the cluster pain) using a custom built olfactometer. The visual stimulus was presented via a beamer-mirror system that was also used for presentation of the reaction task and the rating procedure. Presentation software was used for stimulus presentation, timing and logging of experimental data.
Image acquisition
MRI images were acquired on a 3 T scanner (Siemens TIM TRIO) using a 32-channel head coil. All participants lay supine on the back in the scanner. Echoplanar images were recorded via a protocol optimized for high resolution brainstem imaging (voxel sizes 1.25 × 1.25 × 2.5 mm3, TR 2.61 s, TE 27 ms, 38 axial slices, FoV 216 × 108 mm2, matrix: 172 × 86, flip angle 80°, GRAPPA-accelerated). High resolution T1 weighted images were acquired using an MPRAGE sequence (voxel size 1 × 1 × 1 mm3, TR 2.3 s, TE 2.98 ms, FoV 192 × 256 × 240 mm3, slice orientation: sagittal, flip angle 9°, inversion time 1.1 s).
Preprocessing
Preprocessing was performed using SPM 12. Preprocessing steps included slice timing, image realignment, spatial normalization and warping into MNI space via a segmentation-normalization sequence consisting of co-registration of the structural images to the mean functional image, segmentation of the structural images and normalisation of functional images using segmentation parameters of the structural image of each subject. After normalization, images were smoothed using a 4 mm full width at half maximum Gaussian kernel.
Flipping
Cluster headache patients were stimulated on the site of the cluster. Accordingly, to make the stimulation site homonymous, images of the right sided cluster-headache patients were left-right flipped to match the stimulation site of the other patients using the in-built function fslswapdim of FSL.
Physiological denoising
Heart- and breathing rate were recorded via a respiratory belt and pulsoxymetry and included in the first level analysis as nuisance regressors using the selective averaging method of Deckers et al., that has in detail been described elsewhere [14].
Statistical analysis
1st level
Statistical analysis was done using SPM12 and Matlab version R2014b. First level general linear model included 3 sessions with 4 experimental regressors: ammonia, rose odour, air and checkerboard stimulation. Button presses, anticipation phase, 12 movement regressors (realignment parameters) and 18–20 physiological regressors of cardiac and breathing state were included as nuisance regressors. In case of the gaseous stimuli, a delta function at event-onset was convolved with the hemodynamic response function. The checkerboard was modelled using a box-car function with a duration of 4 s.
2nd level
For the second level analysis, the first level pain-contrast images (ammonia > air) of the individual participants were entered into an Anova consisting of the following groups: inactive cluster headache (out of bout), active cluster headache (ECH in bout and CCH) and healthy controls. Results were generally regarded significant at p < 0.05, corrected for multiple comparisons using the family wise error rate (FWE). As the posterior hypothalamus was a predefined region of interest, we performed a small volume correction using a 6 mm sphere around the peak coordinates reported for the posterior hypothalamus by Maniyar et al. transferred to the left side (− 6, − 6, − 12) [15], to which FWE-correction was restricted.
Results
Fifty-five participants where included in the final analysis (21 episodic cluster headache patients out of bout, 16 active cluster headache patients (10 eCH in bout, 6 CCH) and 18 control participants). Three episodic cluster headache patients were scanned twice: in- and outside the active period. Among the active group, 9 patients took prophylactic medication balanced by 6 patients within the inactive group. Details can be found in Table 1. Statistical analysis of the intensity and unpleasantness ratings of the ammonia-stimulus revealed no statistically significant differences between the three groups. A plot of intensity and unpleasantness of the ammonia stimulus can be found in Fig. 1.
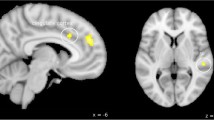
Following trigeminonociceptive stimulation with ammonia (ammonia > air), we found in the functional imaging data a significantly stronger activation within the posterior hypothalamus (x = − 4, y = − 11, z = − 11, T = 3.80, p < 0.05, small-volume-FWE-corrected) in episodic cluster headache patients out of bout when compared to controls. When looking at the contrast estimates of the pain contrast, active cluster headache patients (CCH and ECH in bout) where in between the two other groups but did not differ significantly from either. Figure 2 shows a T-score map of the contrast eCH out of bout vs. controls and a plot of contrast estimates of the 3 groups.
T-score map of the second level anova, contrast inactive cluster headache > controls. Visualization threshold p < 0.001. Significant activation (p < 0.05, small-volume-FWE-corrected) was observed within the posterior hypothalamus ipsilateral to the site of cluster headache and trigeminal nociceptive stimulation (x = − 4, y = − 11, z = − 11, T = 3.80, k = 12 voxels). The bar plot indicates contrast estimates and 95%-confidential intervals for the three groups at the maximum of posterior hypothalamic activation
Discussion
The posterior part of the hypothalamus has been shown to be activated during acute cluster headache attacks in various studies, starting with early H2O-PET studies and continuing to recent fMRI studies [1,2,3, 16,17,18]. To date, no studies exist which investigated cluster headache patients using task related designs in functional imaging. Our data of 37 patients using trigeminal nociceptive input as a task (but outside the cluster headache attack) corroborate hypothalamic involvement in cluster headache pathophysiology. We note that trigeminal nociceptive input activates the posterior part of the hypothalamus, a brain area which is associated with cluster headache pathophysiology [19], and not the hypothalamus proper. Whilst it is undoubted that it is indeed crucial for cluster headache pathophysiology [20], the correct anatomical denomination is a much discussed issue [21,22,23]. For the sake of convenience we keep the wording of the original report [2] and consistently refer to this area as posterior hypothalamus. Regarding the site of activation, our data are in line with previous studies showing the activation ipsilateral to the site of cluster headache [2, 16]. However, different from what we expected, hypothalamic activity levels following trigeminal nociceptive stimulation were highest in patients out of bout, i.e. with inactive cluster, and not within the active group. As there were patients taking medication within both cluster groups, it is rather unlikely that the difference we describe is simply due to an effect of medication. The hypothalamus is involved in processing and modulation of painful trigeminal stimuli via various fiber connections of different hypothalamic nuclei to the spinal trigeminal nucleus, the PAG, the posterior Raphe nucleus and others [10, 18]. Additionally, there are efferent fiber connections of the spinal trigeminal nucleus that directly activate hypothalamic nuclei [19] and the hypothalamus has been shown to be activated in response to trigeminonociceptive stimulation in healthy subjects [10]. It is thus strategically positioned for integrating painful stimuli with signals of energy homeostasis and circadian rhythmicity. Cyclic activity changes within the hypothalamus might constitute the neuronal basis for the beginning of the cluster bout and especially the initiation of a cluster headache attack [24]. Since the posterior hypothalamus is thus frequently activated in active cluster headache (eCH in bout and cCH), these frequent episodes of activation might lead to neurotransmitter exhaustion and hypo-excitability of this area to external stimuli. To our knowledge there are to date no studies directly investigating hypothalamic responses to external stimuli in cluster headache patient. One behavioral measure often discussed to be influenced by hypothalamic activity is the nociceptive blink reflex. Studies point towards altered habituation of the blink reflex in cluster headache patients in the bout and in one study also out of bout [7, 25, 26], but findings are not homogeneous and partly contradicting [27]. Hypothalamic involvement in this mechanism is therefore likely but not proven. The alterations in blink reflex habituation might thus point towards altered hypothalamic functioning in cluster headache but can not help us to explain the current results since other trigeminonociceptive sites of conduction might be involved in mediating blink reflex habituation. The theory of a hypothalamic exhaustion and hypoexcitability in active cluster headache is thus a viable explanation although there is currently no direct experimental evidence to support it. It could account for the fact that, other than expected, hypothalamic activation following nociceptive stimulation of the nasal mucosa was not highest within the active cluster group but within the group of inactive episodic cluster headache patients.
Conclusions
Our data suggest that the posterior hypothalamus might be hyperexcitable in cluster headache patients but only outside the bout while excitability (to external nociceptive stimuli) decreases during in bout periods, possibly due to frequent hypothalamic activation during cluster attacks.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to national data protection acts. Completed analyses might be made available from the corresponding author on reasonable request.
References
Morelli N, Rota E, Gori S et al (2013) Brainstem activation in cluster headache: an adaptive behavioural response? Cephalalgia 33:416–420. https://doi.org/10.1177/0333102412474505
May A, Bahra A, Büchel C et al (1998) Hypothalamic activation in cluster headache attacks. Lancet 352:275–278. https://doi.org/10.1016/S0140-6736(98)02470-2
May A, Bahra A, Büchel C et al (2000) PET and MRA findings in cluster headache and MRA in experimental pain. Neurology 55:1328–1335
Yang F-C, Chou K-H, Fuh J-L et al (2014) Altered hypothalamic functional connectivity in cluster headache: a longitudinal resting-state functional MRI study. J Neurol Neurosurg psychiatry jnnp-2014-308122. https://doi.org/10.1136/jnnp-2014-308122
Qiu E, Tian L, Wang Y et al (2015) Abnormal coactivation of the hypothalamus and salience network in patients with cluster headache. Neurology 84:1402–1408. https://doi.org/10.1212/WNL.0000000000001442
Perrotta A, Serrao M, Ambrosini A et al (2013) Facilitated temporal processing of pain and defective supraspinal control of pain in cluster headache. Pain 154:1325–1332. https://doi.org/10.1016/j.pain.2013.04.012
Holle D, Gaul C, Zillessen S et al (2012) Lateralized central facilitation of trigeminal nociception in cluster headache. Neurology 78:985–992. https://doi.org/10.1212/WNL.0b013e31824d58ce
Stankewitz A, Voit HL, Bingel U et al (2010) A new trigemino-nociceptive stimulation model for event-related fMRI. Cephalalgia 30:475–485. https://doi.org/10.1111/j.1468-2982.2009.01968.x
Stankewitz A, Aderjan D, Eippert F, May A (2011) Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 31:1937–1943. https://doi.org/10.1523/JNEUROSCI.4496-10.2011
Schulte LH, Sprenger C, May A (2015) Physiological brainstem mechanisms of trigeminal nociception: an fMRI study at 3T. Neuroimage 124:518–525. https://doi.org/10.1016/j.neuroimage.2015.09.023
Schulte LH, May A (2016) The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 139:1987–1993. https://doi.org/10.1093/brain/aww097
Schulte LH, Allers A, May A (2017) Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology. https://doi.org/10.1212/WNL.0000000000003963
Kröger IL, May A (2015) Triptan-induced disruption of trigemino-cortical connectivity. Neurology 84:2124–2131. https://doi.org/10.1212/WNL.0000000000001610
Deckers RHR, van Gelderen P, Ries M et al (2006) An adaptive filter for suppression of cardiac and respiratory noise in MRI time series data. Neuroimage 33:1072–1081. https://doi.org/10.1016/j.neuroimage.2006.08.006
Maniyar FH, Sprenger T, Monteith T et al (2014) Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 137:232–241. https://doi.org/10.1093/brain/awt320
Sprenger T, Boecker H, Tolle TR et al (2004) Specific hypothalamic activation during a spontaneous cluster headache attack. Neurology 62:516–517
Morelli N, Pesaresi I, Cafforio G et al (2009) Functional magnetic resonance imaging in episodic cluster headache. J Headache Pain 10:11–14. https://doi.org/10.1007/s10194-008-0085-z
Ferraro S, Nigri A, Bruzzone MG et al (2019) Cluster headache: insights from resting-state functional magnetic resonance imaging. Neurol Sci. https://doi.org/10.1007/s10072-019-03874-8
May A, Schwedt TJ, Magis D et al (2018) Cluster headache. Nat Rev Dis Primers 4:18006. https://doi.org/10.1038/nrdp.2018.6
Hoffmann J, May A (2018) Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol 17:75–83. https://doi.org/10.1016/S1474-4422(17)30405-2
Matharu M, May A (2008) Functional and structural neuroimaging in trigeminal autonomic cephalalgias. Curr Pain Headache Rep 12:132–137
Akram H, Miller S, Lagrata S et al (2016) Ventral tegmental area deep brain stimulation for refractory chronic cluster headache. Neurology 86:1676–1682. https://doi.org/10.1212/WNL.0000000000002632
Matharu MS, Zrinzo L (2010) Deep brain stimulation in cluster headache: hypothalamus or midbrain tegmentum? Curr Pain Headache Rep 14:151–159. https://doi.org/10.1007/s11916-010-0099-5
May A (2005) Cluster headache: pathogenesis, diagnosis, and management. Lancet 366:843–855. https://doi.org/10.1016/S0140-6736(05)67217-0
Coppola G, Di Lorenzo C, Bracaglia M et al (2015) Lateralized nociceptive blink reflex habituation deficit in episodic cluster headache: correlations with clinical features. Cephalalgia 35:600–607. https://doi.org/10.1177/0333102414550418
Perrotta A, Serrao M, Sandrini G et al (2008) Reduced habituation of trigeminal reflexes in patients with episodic cluster headache during cluster period. Cephalalgia 28:950–959. https://doi.org/10.1111/j.1468-2982.2008.01631.x
Holle D, Zillessen S, Gaul C et al (2012) Habituation of the nociceptive blink reflex in episodic and chronic cluster headache. Cephalalgia 32:998–1004. https://doi.org/10.1177/0333102412453955
Acknowledgements
None
Funding
This work was supported by the German Research Foundation, SFB936/A5.
Author information
Authors and Affiliations
Contributions
LHS: data collection, data analysis and interpretation, writing and revising the manuscript. AAH: data collection, writing and revising the manuscript. AM: drafting of the study, writing and revising the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The study was approved by the local ethics committee, Chamber of Physicians, Hamburg, Germany, reference number PV4762.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schulte, L.H., Haji, A.A. & May, A. Phase dependent hypothalamic activation following trigeminal input in cluster headache. J Headache Pain 21, 30 (2020). https://doi.org/10.1186/s10194-020-01098-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-020-01098-2