Abstract
Background/Aim
Migraine-related intracerebral white matter lesions (WMLs) are likely to be microvascular in nature and can be found in all hemispheric lobes. The aim of this study was to investigate migraine patients with or without WMLs to see the effects of these tissue damages on cortical thickness and volume. The role of migraine characteristics (duration of headache, attack frequency, estimated lifetime attack number, aura) was also tested.
Methods
As study participants, 161 female migraine patients (63 with aura; 52 with WMLs) and 40 age-matched healthy female subjects were enrolled in the study. None of the included migraine patients’ headache or aura (where present) was unilaterally side-locked. Patients and controls were all right handed. Except for migraine, patients were free of any medical comorbidity. Cortical reconstruction and segmentation were performed on the 3D T1-weighted images using Freesurfer 5.3 image analysis suite. The automatic cortical parcellation was based on Freesurfer’s Desikan–Killiany–Tourville atlas, which has 31 cortical regions per hemisphere. The segmented regions were divided into five lobes (frontal, parietal, temporal, occipital, insula). Since the left and right differences in lobar and insular volumes/thicknesses were not different among our groups, volume and cortical thickness were calculated for corresponding bilateral lobes.
Results
There was no significant difference in age between the whole migraine and the control groups. Migraineurs with WMLs (L+ patients) were significantly older than lesion-free (L-) patients (P = 0.0003) and controls (P = 0.018). Disease duration (P = 0.003), the total number of migraine attacks (P = 0.022) and the rate of aura (P = 0.0003) were significantly higher in L+ patients than in L- patients. Cortical thickness and volume measurements of lobes were not statistically different between the three groups (L+, L-, control). Age showed a significant negative association with both thickness and volume in each examined lobe (P < 0.001). Intracranial volume (ICV) showed a significant positive association with all regional volumes (P < 0.001). There were no significant group*age, group*ICV, or age*ICV interactions. None of the migraine characteristics were selected by stepwise linear regression as significant predictors of cortical thickness or volume. Only age (for both thickness and volume) and ICV (for volume) were identified as significant predictors (P < 0.001). When the L + group was divided into two subgroups by median split of total and lobar lesion number and volume, the cortical measures did not show any significant difference between the groups with low vs. high lesion number/volume by stepwise linear regression.
Conclusions
In a female migraine group, we found that the WMLs and clinical migraine characteristics have no effect on cortical thickness and volume of bilateral lobes. Lobar cortical thicknesses were equivalent within the range of ±0.1 mm. Only age and ICV proved to be significant predictors; the former for both cortical thickness and volume, while the latter for cortical volume.
Similar content being viewed by others
Introduction
Cortical thickness is both a marker of neurological development and a reflection of cortical function [1, 2]. The cerebral cortex contains high neuronal density, and its thickness varies from 1.5 mm to 5 mm [3]. Both the pyramidal neurons and the interneurons travel through the white matter within the hemisphere during prenatal brain development, and both types of cortical neuronal cells receive projection fibers from the thalamus, and association and commissural fibers from other cortical areas [3].
Migraine is a primary headache disorder [4] that may cause structural and functional alterations in the cerebral cortex [5,6,7,8]. Migraine-related intracerebral white matter lesions (WMLs) are likely to be microvascular in nature and can be found in all four lobes implicating the deep white matter, the subcortical, the periventricular and the callosal commissure locations [9,10,11]. Based on the above mentioned data, we hypothesized that the WMLs – areas of focal axonal and glial cell (astrocyte, oligodendrocyte, microglia) injuries in association with decreased intracellular energy metabolism due to impairment of mitochondria – may cause cortical changes in migraine. For that reason, we investigated migraine patients with or without WMLs to assess the effects of these tissue damages on cortical thickness and volume. In this respect, the potential role of migraine characteristics was tested, as well. Female patients were selected, because migraine is much more prevalent in adult women than men [12] and to avoid the gender-related differences (e.g., longer headache duration, higher intensity of attacks, more frequent nausea, phonophobia and photophobia in women) existing between women and men [13].
Methods
Subjects
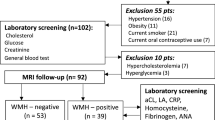
Between 2010 and 2017, a total of 161 female patients fulfilling the International Headache Society (IHS) classification criteria for migraine with or without aura [4] were prospectively screened from the Outpatient Headache Clinic of the Department of Neurology, Medical School, University of Pécs, Hungary. At the time of the study period, all migraineurs had recurrent headaches, and none of them were on chronic prophylactic therapy. For acute migraine treatment, eletriptan, sumatriptan, ibuprofen, diclofenac, acetylsalicylic acid and/or acetaminophen were utilized. The demographic and clinical data of migraineurs were the following: mean age 39.3 ± 12.5, range 18–73 years; disease duration 15.6 ± 11.9, range 1–57 years; attack frequency/month 5.6 ± 4.5, range 0.2–14.8; total number of estimated lifetime migraine attacks (average monthly attack number × 12 × number of migraine disease years to date) 966 ± 1158, range 12–6840; n = 52 with WMLs (L+ patients); n = 63 with aura (Table 1). Migraineurs had no other types of headaches. None of the included migraine patients’ headache or aura was unilaterally side-locked in nature. Magnetic resonance imaging (MRI) was performed in a headache-free period for each patient. Medical comorbidities that could influence migraine characteristics or lead to the formation of WMLs were excluded (hypertension, diabetes mellitus, kidney disease, hepatopathy, high LDL-cholesterol, hyperuricemia, elevated CRP level, thyroid gland disease, systemic autoimmune disease, smoking, cardiac source of embolism, obesity). Based on self-report, all migraineurs were right handed. As controls, 40 age-matched healthy female subjects were included (mean age 38.3 ± 10.0, range 19–66 years, Table 1). Controls were recruited by family physicians in Baranya County, Hungary. Similar to migraine patients, all controls were right handed. All control subjects were free of headache, and their brain MRI studies did not show any structural abnormalities.
MRI acquisition
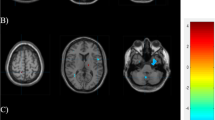
All subjects were scanned on the same 3 T MRI scanner (Magnetom TIM Trio, Siemens AG, Erlangen, Germany) using a 12-channel head coil. The MRI measurements of all patients were performed in a headache-free period. Whole-brain 3D magnetization-prepared rapid gradient-echo (MPRAGE) was acquired using the following parameters: TR/TI/TE = 1900/900/3.4 ms; bandwidth = 179 Hz/px; flip angle = 9°; FOV = 210 × 240 mm2, matrix size = 224 × 256, slice thickness = 0.94 mm, 176 axial slices. Beyond the routine T1- and T2-weighted measurements the scanning protocol also included 2D turbo spin-echo fluid-attenuated inversion recovery (FLAIR) imaging (TR/TI/TE = 13,200/2600/100 ms; bandwidth = 401 Hz/px; echo trains = 14; FOV = 186 × 220 mm2, matrix size = 162 × 192, slice thickness = 1.5 mm, 100 axial slices). WML was considered if visible as hyperintensity on T2-weighted and FLAIR MRI but without hypointensity on T1-weighted MRI and larger than 3 mm, appearing in at least two consecutive slices [14].
MR image analysis
Supratentorial WMLs were marked manually on the FLAIR images using 3D Slicer software ( http://www.slicer.org , Version 4.6.2). An example of WML is displayed on Fig. 1. Total and lobar numbers/volumes of WMLs were calculated for each subject. The borders of lobes were defined as previously described [10].
Cortical reconstruction and segmentation were performed on the T1-weighted 3D MPRAGE images using Freesurfer 5.3 image analysis suite (https://surfer.nmr.mgh.harvard.edu/fswiki). The details of the image processing pipeline are described in prior publications [15, 16]. Quality control was performed throughout the automatic processing stream. When the reconstruction was inaccurate, error correction was performed based on the recommended workflow (http://surfer.nmr.mgh.harvard.edu/fswiki/RecommendedReconstruction).
The automatic cortical parcellation was based on Freesurfer’s “Desikan–Killiany–Tourville” (DKT) atlas [17], which has 31 cortical regions per hemisphere. The segmented regions were divided into four lobes, and surface-area weighted average cortical thickness was calculated for each lobe using the following equation:
where “Area” means the surface area and 1..n is the index of left and right hemispheric cortical regions included in the given lobe. For the definition of frontal, parietal, temporal and occipital lobes please see Table 2. Lobar volumes were calculated as the sum of regional volumes from both hemispheres. Beyond the four lobes, insula was also investigated as the fifth hemispheric lobe of the brain [18]. Insular volume was defined as the sum of left and right insular volumes and mean insular thickness was calculated by averaging the thickness of the left and right insula. Cortical thicknesses and volumes were calculated from both hemispheres because of the following reasons: (i) we had no hypothesis on lateralized effects of migraine attacks (i.e., no migraine patient with unilateral side-locked headache or migraine aura), (ii) the left and right differences in lobar and insular volumes/thicknesses (i.e., lateralities) were not different among our three groups (see the Results section), (iii) we hypothesized a similar degree of white matter damage in the two hemispheres based on an earlier study reporting no differences between the left and right hemispheres in the number of WML, total WML volume, and average WML size in migraine [19].
Statistical analysis
Statistical analyses were performed using SPSS 20.0 software (IBM Corp., Armonk, NY). Differences in age between the whole migraine group and healthy controls were assessed by the Mann-Whitney U-test. Age differences among migraine subgroups (L+ and L-) and the healthy control group were assessed by Kruskal–Wallis test followed by pair-wise comparison using the Mann–Whitney U-test. Continuous migraine-related variables (i.e., disease duration, migraine attack frequency, total number of migraine attacks) were compared between migraine subgroups (L+ and L-) via the Mann–Whitney U-test, while differences in the rate of aura between the same subgroups were assessed using Fisher’s exact test. The left and right differences in lobar and insular volumes/thicknesses (i.e., lateralities of volumes/thicknesses) were compared between our three groups (L+, L-, control) using ANOVA. Cortical thickness and volume differences between the three groups (L+, L-, control) were assessed using ANCOVA with age as covariate for the thickness, and age and total intracranial volume (ICV) as covariates for the volume. Concentrating on the whole migraine group, the possible effects of migraine characteristics (i.e., disease duration, migraine attack frequency, total number of migraine attacks, aura) on cortical thickness and volume were tested using stepwise multiple linear regression analyses. In these models age (for both thickness and volume) and ICV (for volume) were also included as possible predictors.
Concentrating on migraine subgroup with lesion (L+), we assessed the potential effects of lesion number/volume on lobar and insular thicknesses/volumes. Unfortunately, all of our total and lobar lesion number/volume data were definitely not normally distributed (right-skewed with several extreme values). In order to perform powerful statistical analyses, L+ group was divided into patients with mild and patients with moderate-severe lesions. The division was performed based on the median split of whole brain as well as each lobar lesion number/volume, thereby creating binarized subgroup variables: e.g., subgroup with low (below or equal to the median) vs. subgroup with high number of frontal lobe lesions. Since lesions were rare in the occipital lobe with a median lesion number of 0, the division based on lesion number/volume of this lobe was not performed. The effects of these subgroup variables on the insular and lobar thicknesses/volumes were tested using stepwise multiple linear regression analyses, including subgroup variables from the whole brain (for both insular and lobar thicknesses and volumes) and from the same lobe as that of dependent variable (for the lobar thicknesses volumes), age (for both thicknesses and volumes) and ICV (for volumes) as possible predictors. The level of statistical significance was set as < 0.05.
Results
There was no significant difference in age between the whole migraine (including both L+ and L- patients) and the control groups (P = 0.738). The Kruskal–Wallis test revealed significant age differences among migraine subgroups (L+ and L-) and controls (P = 0.001). Post-hoc testing indicated that L+ subgroup was significantly older than the L- (P = 0.0003) and the control (P = 0.018) groups (Table 1). Disease duration (P = 0.003), the total number of migraine attacks (P = 0.022) and the rate of aura (P = 0.0003) were also significantly higher in L+ patients than in L- patients (Table 1).
The left and right differences in lobar and insular volumes/thicknesses (i.e., lateralities) were not different among our groups (L+, L-, control); P = 0.626, 0.965, 0.425, 0.859 and 0.989 for the frontal, parietal, temporal, occipital and insular thicknesses and P = 0.598, 0.252, 0.855, 0.732 and 0.136 for the frontal, parietal, temporal, occipital and insular volumes, respectively. Cortical thickness and volume measurements of the five lobes were not statistically different among our three groups (L+, L-, control), (Table 3.). For both thickness and volume, the mean differences between our groups and the Bonferroni corrected 95% confidence intervals of mean differences are presented in Table 4.
Age showed a significant negative association with both thickness and volume in each examined lobe (P < 0.001). Intracranial volume showed a significant positive association with the volumes of all regions (P < 0.001). There were no significant group*age, group*ICV or age*ICV interactions in the performed analyses.
In the whole migraine group, none of the migraine characteristics were selected by stepwise linear regression as significant predictors of cortical thickness or volume. Only age (for both thickness and volume) and ICV (for volume) were identified as significant predictors (P < 0.001).
Focusing on the L+ patients, none of the binarized total or lobar lesion number/volume variables were selected by stepwise linear regression as significant predictors of the insular or lobar thicknesses/volumes. The main features of WMLs in the L+ group are presented in Table 5.
Discussion
In this study, we investigated a homogeneous (female migraineurs without medical comorbidities) migraine group to explore the potential effects of WMLs and migraine characteristics on cortical lobar thickness and volume. The WMLs and clinical characteristics failed to show any effects on the lobar cortical measures. When the lesion + group was divided into two subgroups by median split of total and lobar lesion number and volume, the cortical measurements (thickness and volume) did not show any significant difference between the groups with low vs. high lesion number/volume by stepwise linear regression. Only age and ICV proved to be significant predictors; the former for both cortical thickness and volume, while the latter for cortical volume.
The lack of the impact of WMLs on cortical thickness and volume may be the consequence of the not reaching the critical size of injured white matter territory, the intralobar separations of lesions with differences in distributions, or less severe intralesional tissue damage. Although the clinically silent brain WMLs are predominantly progressive in nature, smaller lesions may improve in size or even disappear [10]. In addition, the normal-appearing white matter (NAWM) did not show MRI signs of tissue injury in migraine patients with or without WML [9].
The negative association of age with cortical thickness and volume raises the possibility that WMLs are age-related. WMLs can develop at any age during the active migraine years, and their presence does not correlate with age [20,21,22,23]. Disease duration and attack frequency are the main indicators for brain damage in migraine [22, 24]. Usually, ageing associates with longer disease duration and higher lifetime attack number, and these factors may increase the risk of oxidative stress-related endothelial injury and atherosclerosis [11]. Furthermore, a wide range of vascular risk factors contribute to lesion formation [22, 25]. In the present study, both migraineurs and controls lacked any medical comorbidity, thus the role of ageing in lesion development is less likely.
Previous studies presented several different cortical regions with morphological abnormalities, sometimes with contradictory findings. Due to differences in study aims, morphometric MRI studies, analytical approaches and number of study participants, our results are not directly comparable to earlier studies [6,7,8, 26,27,28,29,30,31,32,33,34,35,36].
Some of these studies used a voxel-based morphometry (VBM) approach [7, 8, 26, 27, 29, 32, 36], which analyzes the brain on a voxel by voxel basis, while our approach examines brain changes at a larger territory level (i.e., lobar level). In addition, several other methodological differences exist between VBM and our surface-based analysis. VBM does not distinguish between different cortical morphological properties and various methodological factors, including cortical thickness, surface area, cortical volume, gyrification pattern, T1 signal alterations within a physiologic range, registration artefacts and smoothing [37,38,39]. Moreover, the significant group differences reported by a VBM analysis are not necessarily homogenous in terms of the underlying factors [37]. Based on these differences, VBM and our method should be considered as complementary techniques [38].
Others used surface-based analysis to examine local cortical thickness changes in migraine (6,28,30,31,33–35). Most of these studies were not interested in the direct thickness differences between migraineurs and controls, but rather examined between-hemisphere cortical differences related to the headache side [6], interregional cortical thickness correlations to differentiate groups of migraine patients from healthy controls [34], and differences in cortical thickness-to-pain threshold correlations between migraineurs and controls [33]. Another study reported significantly decreased left anterior midcingulate cortical thickness in the migraine group, but the number of subjects was low (17 migraine patients vs. 18 controls) [31]. From the largest studies, one reported no significant cortical thickness differences at all [28], while the other found significant differences between migraineurs and controls in several small brain regions [30]. The latter should be interpreted in the context of statistically controlling for the whole-hemisphere average cortical thickness during vertex-wise statistical analysis of thickness. Since the overall average cortical thickness was significantly increased in the migraineurs, such correction may add noise and provide inaccurate data. A vertex-based study conducted on females found higher cortical thickness in the superior frontal gyrus, paracentral gyrus, temporal pole, precuneus and lower cortical thickness in the anterior cingulate of migraineurs [25]. Interestingly, the authors were unable to detect the well-established cortical atrophy with advancing age [40] for the insula in the migraine group, while we could demonstrate it by measuring both cortical thickness and cortical volume without any group*age interaction. Two recent studies investigated only migraine patients with aura [41, 42]. One of them found slightly thicker cortical visual areas in female migraineurs with aura [41], while the other found no solid evidence for cortical thickness differences between patients with aura and controls [42]. The second study found reduced volume of the left fusiform gyrus in migraineurs with aura compared to controls. We conducted a region-of-interest (ROI) surface-based analysis, which has unique strengths and limitations compared to the vertex-wise surface-based method (e.g., ROI analysis does not need smoothing or inter-subject registration, and the problem of multiple comparisons is substantially reduced compared to a vertex-wise comparison). The measurement at a single vertex is often quite noisy, which may reduce the statistical power [43]. However, if the structural differences cross the boundaries of the predefined ROIs, then only the vertex-wise approach may find them [44]. Moreover, the vertex-wise approach doesn’t need an a priori hypothesis. From the above cited studies, only Datta et al. [28] and Gaist at al [41]. used a similar approach to our one, and although the results of Datta et al. [28] were also negative, the investigated ROIs were quite different in both of these studies compared to the present study.
In one study the cortical thickness measures by Freesurfer agreed to those obtained using traditional neuropathologic techniques within 0.2 mm (with a mean difference of 0.077 mm) [45]; in another study the accuracy was better than 0.5 mm compared to manual measures on MRI data [46], while test-retest within-scanner error in local cortical thickness measurement was found to be about 0.12 mm in average [47]. Based on the order of these numbers, in the present study, only cortical thickness differences of at least 0.1 mm was considered scientifically relevant. Since our Bonferroni corrected 95% confidence intervals of the cortical thickness differences were within the range of − 0.059 to + 0.082 mm (Table 4) for each lobe and each subgroup comparison, equivalence testing suggests that mean lobar cortical thicknesses of all three groups are equivalent within the pre-defined practically relevant limits (i.e., ± 0.1 mm).
Strengths and limitations
The main strengths of our study include the relatively large single center sample size, assessing both cortical thickness and volume, and performing equivalence testing. Our study was cross-sectional, where the detection of subtle cortical changes is hindered by inter-individual differences. Longitudinal studies eliminating much of these differences are also needed to further support that no cortical changes occur in migraine. Instead of searching for very subtle structural differences, our main goal was to examine whether migraine lesions cause thickness/volume changes at the level of larger cortical territories (i.e., lobar level). This approach is useful to detect more robust lobar changes even if there are individual variations of morphometric changes within the lobes. However, we acknowledge that very subtle structural changes with consistent anatomical locations can exist, which may be detected by vertex-wise analysis or more detailed ROI analysis, while such abnormalities may be averaged out and overlooked when examining at the lobar level.
Conclusions
In summary, we investigated a female migraine group, and found that neither the lesions nor other clinical characteristics have a detectable effect on cortical thickness and volume of bilateral intracerebral lobes. Cortical thicknesses were equivalent within the range of ±0.1 mm. Only age and ICV proved to be significant predictors; the former for both cortical thickness and volume, while the latter for cortical volume.
Abbreviations
- ANCOVA:
-
Analysis of covariance
- ANOVA:
-
Analysis of variance
- DKT:
-
Desikan–Killiany–Tourville
- FLAIR:
-
Fluid-attenuated inversion recovery
- FOV:
-
Field-of-view
- ICV:
-
Intracranial volume
- IHS:
-
International Headache Society
- MPRAGE:
-
Magnetization-prepared rapid gradient-echo
- MRI:
-
Magnetic resonance imaging
- NAWM:
-
Normal-appearing white matter
- ROI:
-
Region-of-interest
- SPSS:
-
Statistical package for the social sciences
- VBM:
-
Voxel-based morphometry
- WML:
-
White matter lesions
References
Jernigan TL, Trauner DA, Hesselink JR, Tallal PA (1991) Maturation of human cerebrum observed in vivo during adolescence. Brain 114:2037–2049
Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB (1996) Brain development, gender and IQ in children. A volumetric imaging study. Brain 119:1763–1774
Cerebrum, chapter 9, pages 350–369. In: Duus’ Topical Diagnosis in Neurology: Anatomy, Physiology, Signs, Symptoms, 4th edition - By M. Baehr and M. Frotscher 2006. ISBN 3–13–612804-4 (GTV), ISBN 1–58890–215-3 (TNY)
Headache Classification Committee of the International Headache Society (IHS) (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Valfrè W, Rainero I, Bergui M, Pinessi L (2008) Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 48:109–117
Hougaard A, Amin FM, Hoffmann MB, Larsson HB, Magon S, Sprenger T, Ashina M (2015) Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia 35:3–9
Jia Z, Yu S (2017) Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin 14:130–140
Neeb L, Bastian K, Villringer K, Israel H, Reuter U, Fiebach JB (2017) Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache 57:400–416
Aradi M, Schwarcz A, Perlaki G, Orsi G, Kovács N, Trauninger A, Kamson DO, Erdélyi-Bótor S, Nagy F, Nagy SA, Dóczi T, Komoly S, Pfund Z (2013) Quantitative MRI studies of chronic brain white matter hyperintensities in migraine patients. Headache 53:752–763
Erdélyi-Bótor S, Aradi M, Kamson DO, Kovács N, Perlaki G, Orsi G, Nagy SA, Schwarcz A, Dóczi T, Komoly S, Deli G, Trauninger A, Pfund Z (2015) Changes of migraine-related white matter hyperintensities after 3 years: a longitudinal MRI study. Headache 55:55–70
Erdélyi-Bótor S, Komáromy H, Kamson DO, Kovács N, Perlaki G, Orsi G, Molnár T, Illes Z, Nagy L, Kéki S, Deli G, Bosnyák E, Trauninger A, Pfund Z (2017) Serum L-arginine and dimethylarginine levels in migraine patients with brain white matter lesions. Cephalalgia 37:71–580
Vetvik KG, MacGregor EA (2017) Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 16:76–87
Bolay H, Ozge A, Saginc P, Orekici G, Uludüz D, Yalın O, Siva A, Bıçakçi Ş, Karakurum B, Öztürk M (2015) Gender influences headache characteristics with increasing age in migraine patients. Cephalalgia 3:792–800
Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MMB (2003) Silent brain infarcts and white matter lesions increase stroke risk in general population. Stroke 34:1126–1129
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055
Klein A, Tourville J (2012) 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 6:171
Stephani C, Fernandez-Baca Vaca G, Maciunas R, Koubeissi M, Lüders HO (2011) Functional neuroanatomy of the insular lobe. Brain Struct Funct 216:137–149
Kamson DO, Illés Z, Aradi M, Orsi G, Perlaki G, Leél-Őssy E, Erdélyi-Botor S, Pótó L, Trauninger A, Pfund Z (2012) Volumetric comparisons of supratentorial white matter hyperintensities on FLAIR MRI in patients with migraine and multiple sclerosis. J Clin Neurosci 19:696–701
Fazekas F, Koch M, Schmidt R, Offenbacher H, Payer F, Freidl W, Lechner H (1992) The prevalence of cerebral damage varies with migraine type: a MRI study. Headache 32:287–291
De Benedittis G, Lorenzetti A, Sina C, Bernasconi V (1995) Magnetic resonance imaging in migraine and tension-type headache. Headache 35:264–268
Trauninger A, Leél-Ossy E, Kamson DO, Pótó L, Aradi M, Kövér F, Imre M, Komáromy H, Erdélyi-Botor S, Patzkó A, Pfund Z (2011) Risk factors of migraine-related brain white matter hyperintensities: an investigation of 186 patients. J Headache Pain. 12:97–103
Eidlitz-Markus T, Zeharia A, Haimi-Cohen Y, Konen O (2013) MRI white matter lesions in pediatric migraine. Cephalalgia 33:906–913
Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, van Buchem MA (2008) Attack frequency and disease duration as indicators for brain damage in migraine. Headache 48:1044–1055
Sacco S, Cerone D, Carolei A (2008) Comorbid neuropathologies in migraine: an update on cerebrovascular and cardiovascular aspects. J Headache Pain 9:237–248
Matharu MS, Good CD, May A, Bahra A, Goadsby PJ (2003) No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol 10:53–57
Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M (2006) Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 37:1765–1770
Datta R, Detre JA, Aguirre GK, Cucchiara B (2011) Absence of changes in cortical thickness in patients with migraine. Cephalalgia 31:1452–1458
Liu J, Zhao L, Li G, Xiong S, Nan J, Li J, Yuan K, von Deneen KM, Liang F, Qin W, Tian J (2012) Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One 7:e51250
Messina R, Rocca MA, Colombo B, Valsasina P, Horsfield MA, Copetti M, Falini A, Comi G, Filippi M (2013) Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology 268:170–180
Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA (2014) Altered Brain Structure and Function Correlate with Disease Severity and Pain Catastrophizing in Migraine Patients. eNeuro 1:e20 14
Rocca MA, Messina R, Colombo B, Falini A, Comi G, Filippi M (2014) Structural brain MRI abnormalities in pediatric patients with migraine. J Neurol 261:350–357
Schwedt TJ, Chong CD (2014) Correlations between brain cortical thickness and cutaneous pain thresholds are atypical in adults with migraine. PLoS One 9:e99791
Schwedt TJ, Berisha V, Chong CD (2015) Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One 10:e0116687
Maleki N, Barmettler G, Moulton EA, Scrivani S, Veggeberg R, Spierings EL, Burstein R, Becerra L, Borsook D (2015) Female migraineurs show lack of insular thinning with age. Pain 156:1232–1239
Messina R, Rocca MA, Colombo B, Teggi R, Falini A, Comi G, Filippi M (2017) Structural brain abnormalities in patients with vestibular migraine. J Neurol 264:295–303
Palaniyappan L, Liddle PF (2012) Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. NeuroImage 60:693–699
Gerrits NJ, van Loenhoud AC, van den Berg SF, Berendse HW, Foncke EM, Klein M, Stoffers D, van der Werf YD, van den Heuvel OA (2016) Cortical thickness, surface area and subcortical volume differentially contribute to cognitive heterogeneity in Parkinson's disease. PLoS One 11:e0148852
Chung S, Wang X, Lui YW (2017) Influence of T1-weighted signal intensity on FSL voxel based Morphometry and FreeSurfer cortical thickness. AJNR Am J Neuroradiol 38:726–728
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B (2004) Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730
Gaist D, Hougaard A, Garde E, Reislev NL, Wiwie R, Iversen P, Madsen CG, Blaabjerg M, Nielsen HH, Krøigård T, Østergaard K, Kyvik KO, Hjelmborg J, Madsen K, Siebner HR, Ashina M (2018) Migraine with visual aura associated with thicker visual cortex. Brain. https://doi.org/10.1093/brain/awx382 [Epub ahead of print]
Petrusic I, Dakovic M, Kacar K, Zidverc-Trajkovic J (2018) Migraine with Aura: surface-based analysis of the cerebral cortex with magnetic resonance imaging. Korean J Radiol 19:767–776
Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, Rosen B, Fischl B, Knudsen GM (2014) Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. NeuroImage 92:225–236
Greve DN, Van der Haegen L, Cai Q, Stufflebeam S, Sabuncu MR, Fischl B, Brysbaert M (2013) A surface-based analysis of language lateralization and cortical asymmetry. J Cogn Neurosci 25:1477–1492
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002) Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58:695–701
Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B (2003) Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60:878–888
Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B (2006) Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage 32:180–194
Acknowledgements
Not applicable.
Funding
This work was supported by grants from EEA/Norwegian Financial Mechanism HU 0114 -“Save what can be saved” - applied neurological research using high-field magnetic resonance imaging, by TÁMOP-4.2.1/B, EFOP-3.6.2-16-2017-00008, “The role of neuroinflammation in neurodegeneration: From molecules to clinics”, Hungarian Brain Research Program (KTIA_NAP_13-2-2014-0019 and 2017–1.2.1-NKP-2017-00002), Institutional Excellence Program (17886–4/2018/FEKUTSTRAT), ÚNKP-18-4-PTE-315 New National Excellence Program of the Ministry of Human Capacities, PTE ÁOK-KA-2017-05, PTE ÁOK-KA-2017-06 and János Bolyai Research Scholarship of the Hungarian Academy of Sciences (G. P. and G. O.). Z. P. was funded by PTE ÁOK-KA-2017-23. EFOP-3.6.3-VEKOP_00009.
Availability of data and materials
All data and materials are available. All authors take full responsibility for the data and materials.
Author information
Authors and Affiliations
Contributions
Category 1(a) Conception and design ZP, DOK, GP. (b) Acquisition of data ZP, GP, EB, AT. (c) Analysis and interpretation of data GP, GO, SAN, MH, HK. Category 2 (a) Drafting the manuscript ZP, DOK, GP. (b)Revising it for intellectual content (c) ZP, DOK, GP, AT, FJ. Category 3 (a) Final approval of the completed manuscript HK, MH, GP, GO, SAN, EB, DOK, FJ, AT, ZP.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All subjects provided informed consent to study participation. Studies were performed in accordance with the approval (4022) of the Regional Research Ethics Committee of the Medical Center, Pécs.
Consent for publication
Publication is accepted by all authors.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Komáromy, H., He, M., Perlaki, G. et al. Influence of hemispheric white matter lesions and migraine characteristics on cortical thickness and volume. J Headache Pain 20, 4 (2019). https://doi.org/10.1186/s10194-019-0959-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-019-0959-2