Abstract
Background
Urotensin-II (U-II) is a peptide recognized by its potent vasoconstrictor activity in many vascular events, however the role of urotensin-II in migraine has not been considered yet. The molecular mechanisms and genetics of migraine have not been fully clarified yet, but it is well-known that vascular changes considerably contribute in pathophysiology of migraine and also its complications. The aim of this study was to analyze the plasma U-II levels along with genotype distributions and allele frequencies for UTS2 Thr21Met and Ser89Asn polymorphisms among the patients with migraine without aura (MWoA).
Methods
One hundred eighty-six patients with MWoA and 171 healthy individuals were included in this study. Plasma U-II levels were measured in attack free period. The genotype and allele frequencies for the Thr21Met (T21M) and Ser89Asn (S89N) polymorphisms in the UTS2 gene were analyzed.
Results
Plasma U-II levels were significantly higher in MWoA patients (p = 0.002). We detected a significant association between the T21M polymorphism in the UTS2 gene and migraine (53.8 % in patients, 40.4 % in controls, p = 0.035), but not with S89N polymorphism (p = 0.620). A significant relationship was found between U-II levels and MIDAS score (β = 0.508, p = 0.001).
Conclusion
Our study suggests that U-II may play a role in migraine pathogenesis; also Thr21Met polymorphism was associated with the risk of migraine disease. Further studies are needed for considering the role of U-II in migraine pathophysiology and for deciding if UTS2 gene may be a novel candidate gene in migraine cases.
Similar content being viewed by others
Background
Migraine is a common neurological disorder that affects approximately 12 % of the population [1]. However, in recent years, it has been suggested that migraines are formed as a result of neuronal vascular event chains triggered by endogenous and/or exogenous factors in people with a genetic predisposition [2, 3]. Many first-degree relatives of migraine patients have a history of migraines, and twin studies conducted show that migraines have a strong genetic component [4–6].
Urotensin-2 (U-II) is a cyclic peptide composed of 11 amino acids that was first isolated from the goby neurosecretory system in 1969 [7]. The human receptor for U-II (hUT2R) is a G-protein coupled receptor (GPR14) [8]. U-II and its receptor (UTR) are found in different tissues such as the central nervous system, peripheral vascular tissues, the heart, and the kidneys [8, 9]. Clark et al. suggested that UII receptor mRNA and choline acetyltransferase exist together in the mesopontine tegmental area [10]. U-II is a vasoactive substance that has a similar peptide structure to somatostatin and is a more powerful vasoconstrictor than endothelin-1 (ET-1) [11]. The vasoconstrictor effect of U-II is 50 times higher on arteries and 10 times higher on veins than ET-1. U-II is thought to have endothelium-dependent vasodilator and endothelium-independent vasoconstrictor effects, and its net effect may depend on the balance between these two individual effects [12]. However, studies have shown that it plays other physiological roles beyond the regulation of vascular tone and cholinergic activity. The urotensinergic system has been shown to be associated with heart failure, hypertension, diabetes, preeclampsia, renal and liver diseases, neurological and psychiatric disorders [13, 14]. U-II is known to be expressed in the brain and spinal cord. It is recognized as a neuro-mediator in the central nervous system [15, 16].
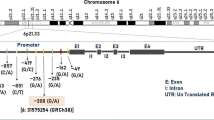
U-II may also play a role in migraine pathogenesis, especially considering its known effects on the central and peripheral nervous system. The U-II gene (UTS2) is located at the 1p36 locus. According to data from the US National Center for Biotechnology Information, more than 60 single nucleotide polymorphisms (SNPs) have been recorded in the human UTS2 gene. Thr21Met (T21M, rs228648) and Ser89Asn (S89N, rs2890565) polymorphisms have been found at high allelic frequencies in Japanese populations [17]; these are the same polymorphisms that were selected for investigation in our study. Although many studies have shown the roles of various genetic factors and polymorphisms in migraine disease, no studies have focused on theT21M and S89N polymorphisms in the UTS2 gene in migraine patients until now.
Therefore, the purpose of this study is to examine the possible relationships between the T21M and S89N polymorphisms in the UTS2 gene and MWoA and to detect the possible role of U-II in the pathogenesis of MWoA by measuring serum U-II levels.
Methods
Study population
Study approval was obtained from the Ethics Committee of the Gaziantep University Faculty of Medicine. Informed consent was obtained from all subjects prior to the study. This study examined 186 consecutive patients aged 18–45 years, who were diagnosed as having migraines, had not been on prophylactic treatment for at least 3 months and had least 72 h migraine attack drug-free period before obtaining blood samples in the interictal phase. Since it is thought to have endothelium-dependent vasodilator and endothelium-independent vasoconstrictor effects, and its net effect may depend on the balance between these two individual effects and we don’t know if it has an effect in migraine attacks we decided to obtain the blood samples in the interictal phase from all of the patients.
Migraines were diagnosed according to the ICHD-2 criteria [18] by experienced neurologists in our clinic and then enrolled into the study. For homogenizing the group, we selected only MWoA patients. The control group (n = 171) was composed of age and gender matched healthy cases that consented to join to the control group.
Cases with history of diabetes mellitus (fasting blood glucose ≥ 120 mg/dl); hypertension (Blood pressure (BP) ≥ 140/90 mmHg); chronic renal failure; liver cirrhosis; any type of cancer; thyroid diseases; alcohol and substance abuse; chronic neurologic illnesses, including epilepsy, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, Wilson’s disease, and previous cerebrovascular and cardiovascular diseases, morbid obesity; and any existing infection were excluded from both groups. The medical histories, physical and neurologic examination findings, and body mass indices (BMI) of all cases were recorded. The migraine patients were questioned regarding the disease duration, the type of migraine, the frequency of migraine attacks (for the last 3 months), drugs used, and smoking. The Migraine Disability Assessment Scale (MIDAS) was applied to measure the extent to which migraine headaches decreased the patients’ standard of living. Routine laboratory examinations, including total blood count, serum electrolytes, serum creatinine, blood urea nitrogen (BUN), fasting blood glucose levels, and liver function tests, were performed in all cases. The glomerular filtration rate (GFR) was calculated according to the Modification of Diet in Renal Disease (MDRD) guidelines [19].
Power analysis
Sample size was estimated using a power calculation based on 0.2 ± 0.6 changes in urotensin between groups. It was estimated that at least 142 participants in each group would be required to detect a significant difference between control and migraine groups at 80 % power level and an alpha error of % 5.
Blood samples and DNA isolation
Venous blood samples were drawn from the antecubital vein in the morning hours after 12 h of fasting and for at least 72 h without symptomatic migraine medication. The plasma was separated from the blood samples by adding EDTA and centrifuging the samples at 1000 g for 15 min. The plasma samples were then stored at -80 °C until U-II levels were measured. Plasma U-II concentrations were measured using a quantitative sandwich-type enzyme immunoassay UT2 kit (Elx 800 ELISA; Cusabio Biotech, Winooski, VT, USA). Genomic DNA extraction was performed from the plasma-free blood pellets using a standard proteinase K and salt precipitation method. The extracted DNA was stored at -20 °C.
SNP genotyping
Samples were genotyped for the UTS2 SNPs using a validated TaqMan SNP Genotyping Assay (Applied Biosystems Inc. (ABI), Foster City, CA, USA) that employed predesigned primers and probes for the UTS2 gene SNPs (T21M, rs228648; S89N, rs2890565) (ABI). One allelic TaqMan probe was labeled with a fluorescent FAM dye, and the other was labeled with a VIC dye. For each polymerase chain reaction (PCR), 5 μL of genomic DNA solution (5 ng/μL) was added to an aliquot of 2× TaqMan universal PCR Master Mix, resulting in primer and probe final concentrations of 180 and 40 nM, respectively. The amplification protocol consisted of the following steps: (1) an initial denaturation at 95 °C for 10 min and (2) 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min, with amplification and fluorescence detection performed using a Qiagen Rotor-Gene Q Real-time PCR system. At least 10 % of the blood samples were run twice in separate assays with a concordance of genotype designation of 100 %.
Statistical analysis
The results are expressed as either means ± the standard deviations (SDs) or the percentage. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software version 20.0 (Inc. Chicago, IL). The chi-squared test was used to calculate significant differences in the genotype and allele frequencies. The unpaired Student’s t test was used to compare the differences between the mean values of the 2 groups. The effects of the genetic polymorphisms on the risk of SSc were estimated with an odds ratio (OR) and 95 % confidence interval (CI). The haplotype analysis was performed using SHEsis software (http://analysis.bio-x.cn/myAnalysis.php). All of the statistical tests and p values were two-sided, and p < 0.05 was considered statistically significant.
Results
One hundred eighty-six patients diagnosed with MWoA and 171 healthy control subjects were enrolled in this study. No significant differences existed between the two groups in terms of gender distribution, age, BMI, or smoking status. A total of 101 (54.3 %) migraine patients were using migraine attack drugs (triptans, nonsteroidal anti-inflammatory drugs (NSAIDs), paracetamol and combination analgesic). The demographic and laboratory characteristics of the study group are shown in Table 1.
Plasma urotensin-2 levels by ELISA assay
The mean U-II levels were 1.19 ± 0.69 pg/ml in the migraine group and 0.97 ± 0.7 pg/ml in the control group. U-II plasma levels were significantly higher in the migraine group (p = 0.002) (Fig. 1). No differences in U-II levels were found in terms of gender, smoking status, symptomatic medication history in either group (Table 2). Disease duration and attack frequency were not significantly correlated with U-II levels (β:0.48/p:0.657, β: 0.195/p:0.175; respectively). A significant relationship was found between U-II levels and MIDAS score. U-II levels were significantly higher in patients with higher MIDAS scores. A 1 unit increase in the MIDAS score resulted in a 0.508 unit increase in U-II levels (β = 0.508, p = 0.001) (Fig. 2).
Genotyping
Genotype and allele frequencies for T21M (rs228648) and S89N (rs2890565) polymorphisms in the UTS2 gene in the patient and control groups are shown in Table 3. We detected a significant association between the T21M polymorphism in the UTS2 gene and migraine but no significant relationship between the S89N polymorphism and migraine in our study (p = 0.620). T21T genotype frequency was more prevalent in the control group (34.4 % in the patients compared with 42.1 % in the controls). T21M genotype frequency was more prevalent in the migraine group (53.8 % in the patients compared with 40.4 % in the controls, p = 0.035) and the According to these results, patients with theT21M genotype are 1.63 times more likely to become migraine patients than patients with the T21T genotype (OR = 1.63, p = 0.035). No significant differences were found in the 21 M polymorphism allele frequencies between the migraine and control groups (p = 0.786). Moreover, no significant relationships were found between the MIDAS scores and the T21M (p = 0.502) or S89N (p = 0.300) polymorphisms in the UTS2 gene in the migraine groups. Finally, no significant relationships were found between the smoking status and the T21M (p = 0.885) or S89N (p = 0791) polymorphisms in the UTS2 gene in the migraine group. There were insignificant increases in MN, MS and TS haplotype frequencies in migraine patients (Table 4).
Relationship between genotypes and expression
The plasma levels of U-II were tended to be higher without statistical significance in TM and MM genotype (p = 0.545). The relationship between plasma U-II protein level and Thr21Met polymorphism in patients was shown in Fig. 3.
Discussion
Our results revealed a significant elevation of serum U-II levels in migraine patients. Additionally, a significant association of migraine with T21M polymorphism but not with S89N polymorphism of urotensin gene was observed in the present study.
Several peptides have been reported in relation with migraine pathophysiology. The role of calcitonin gene-related peptide (CGRP) in migraine pathogenesis is still under investigation in current researches [20, 21]. Pituitary adenylate cyclase-activating polypeptide and substance P (SP) had craniocervical vasodilatation, plasma protein extravasation, peripheral and central sensitization effects in migraine pathogenesis [22]. Serum levels of vasoactive intestinal peptide (VIP); a marker of parasympathetic nervous system, was found to be increased in chronic and episodic migraineurs in attack free period [23]. Diverse results for neuropeptide Y (NPY); a marker of sympathetic nervous system with long-lasting vasoconstrictor effects in cerebral circulation, was reported in migraineurs [22].
U-II and its receptor have many functions, including the regulation of behavior and neuroendocrine activities, cardiovascular tone control, motor activity, the sleep-wake cycle, and hypothalamic-pituitary-adrenal axis control [14, 24–26]. U-II plasma concentrations are low in healthy individuals [13]. Previously some studies have measured higher UT2 peptide levels in various metabolic and cardiovascular diseases such as; chronic heart failure, acute myocardial infarction, hypertension and diabetes mellitus [27–29].
Considerably increased risk of ischemic events, in various organs including brain has already been documented in migraine [30]. Currently the exact mechanisms of that relation between migraine and stroke are not understood entirely. Distinct mechanisms were proposed for migraine-related ischemia that occurs either during the attack or attack-free periods. Additionally, it is not clear yet if stroke is a consequence of migraine or both of the conditions occur due to another shared pathological course. In this case, a genetic link may be responsible for influencing the course and variability of the consequences of migraine [31]. Nevertheless, endothelial dysfunction was proposed as one of the main paths in this association [32, 33].
U-II is a vasoactive peptide with ubiquitous effects in various human body tissues. It has been suggested to have endothelium-dependent vasodilator and endothelium-independent vasoconstrictor effects, with its net effect depending on the balance between these two individual effects [12]. Yet in general, U-II is known to have a powerful vasoconstrictor effect.
As mentioned above, since U-II has dual effect on endothelium through distinct pathways and we don’t have data if it has an effect in migraine attacks or not, the blood samples were obtained in the interictal phase in all of the cases. Therefore, the significantly higher levels of plasma U-II in the present study present only the U-II status in the attack-free period that may be a limitation of the present study. Understanding the effects of U-II on the attacks is yet to be waiting for further investigation. However, we believe that the stated concerns about the dual effect of U-II should be taken under consideration also in further research that aim to investigate the plasma U-II effects in migraine attacks.
We found a significant positive relation between the MIDAS scores and plasma U-II levels in the present study. This finding may indicate that the severity of migraine may be influenced by plasma U-II and in turn it may have a bad effect in quality of life of the cases.
Many first-degree relatives of migraine patients have a history of migraine, and twin studies showed that migraine have a strong genetic component [3–5]. Genetic factors may cause a tendency (predispose) to have migraine attacks. Despite increasing number of studies suggesting a role for genetic factors in the pathogenesis of migraine, the responsible genes have not yet been determined. Despite, neuronal vascular event chains triggered by endogenous and/or exogenous factors in people with a genetic predisposition, it is now believed that neuronal dysfunction is the possible primary reason in the pathophysiology of the disease and vasodilation and vasoconstriction phases are probably epiphenomena [34, 35].
Non-familial migraines can be considered as polygenic when the diversity of both the number and severity of attacks and the duration of the attacks are considered. Various genes were supposed to be involved in migraine pathophysiology. Our results indicate that a significant association exists between the T21M polymorphism and migraine. The T21M genotype patients were 1.63 times more likely to become migraine than patients with the T21T genotype in the present study. Amino acid change upon Thr21Met polymorphism may affect protein folding efficiency or structure. By this way, differently folded protein may be exposed to protein degradation processes more or less than natively folded protein. Also, some amino acid sequences form signal for degradation. With this amino acid change, degradation signal may be created and this causes U-II level to decrease. Moreover, newly formed Met codon on mRNA may provide potential translation starting point for ribosome, although it is a low probability. So, this causes a truncated U-II protein product. All these possibilities may explain U-II level changes upon Thr21Met variant presence. Also, we revealed that the plasma U-II levels tended to be higher in cases with Thr21Met polymorphism as shown in Fig. 3. However the difference couldn’t reach a statistically significant level.
Several publications have indicated a possible relationship between UTS2 gene polymorphisms and hypertension, diabetes mellitus, Behçet’s disease, and systemic sclerosis [17, 36–38]. Recently, genome-wide studies have provided new insights for genes associated with migraine disease (the ion channel gene, TRPM8, FHL5, ASTN2, and LRP1 [39] but UTS2 gene was not one of them. Since, we believe that it is worthy to evaluate this vasoactive peptide in migraine pathophysiology since vascular changes predominantly take place in migraine cases in both attack and attack-free periods.
Conclusion
Our study suggests that U-II may play a role in migraine pathogenesis, also Thr21Met polymorphism was associated with the risk of migraine disease. Further studies are needed for considering the role of U-II in migraine pathophysiology and for deciding if UTS2 gene may be a novel candidate gene in migraine cases.
Abbreviations
- BMI:
-
body mass index
- CGRP:
-
calcitonin gene-related peptide
- CI:
-
confidence interval
- ET-1:
-
endothelin-1
- FHM:
-
familial hemiplegic migraine
- GPR14:
-
G-protein coupled receptor
- hUT2R:
-
human receptor for U-II
- ICHD-3:
-
International Classification of Headache Disorders
- MDRD:
-
Modification of Diet in Renal Disease
- MIDAS:
-
Migraine Disability Assessment Scale
- MWoA:
-
migraine without aura
- NPY:
-
neuropeptide Y
- NSAID:
-
nonsteroidal anti-inflammatory drug
- OR:
-
odds ratio
- S89N:
-
Ser89Asn
- SD:
-
standard deviation
- SP:
-
substance P
- T21M:
-
Thr21Met
- U-II:
-
urotensin-2
- UTR:
-
U-II receptor
- VIP:
-
vasoactive intestinal peptide
References
Lipton RB, Bigal ME (2005) Migraine: epidemiology, impact, and risk factors for progression. Headache 45(Suppl 1):S3–S13. doi:10.1111/j.1526-4610.2005.4501001.x
Demarquay G, Mauguière F (2015) Central nervous system underpinnings of sensory hypersensitivity in migraine: insights from Neuroimaging and electrophysiological studies. Headache. doi:10.1111/head.12651
Fang J, An X, Chen S, Yu Z, Ma Q, Qu H (2015) Case-control study of GRIA1 and GRIA3 gene variants in migraine. J Headache Pain 17(1):2. doi:10.1186/s10194-016-0592-2
de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM (2009) Molecular genetics of migraine. Hum Genet 126(1):115–132. doi:10.1007/s00439-009-0684-z
Schürks M (2012) Genetics of migraine in the age of genome-wide association studies. J Headache Pain 13(1):1–9. doi:10.1007/s10194-011-0399-0
Mulder EJ, Van Baal C, Gaist D, Kallela M, Kaprio J, Svensson DA, Nyholt DR, Martin NG, MacGregor AJ, Cherkas LF, Boomsma DI, Palotie A (2003) Genetic and environmental influences on migraine: a twin study across six countries. Twin Res 6(5):422–431. doi:10.1375/136905203770326420
Onan D, Hannan RD, Thomas WG (2004) Urotensin II: The old kid in town. Trends Endocrinol Metab 15(4):175–182. doi:10.1016/j.tem.2004.03.007
Lavecchia A, Cosconati S, Novellino E (2005) Architecture of the human urotensin II receptor: comparison of the binding domains of peptide and non-peptide urotensin II agonists. J Med Chem 48(7):2480–2492. doi:10.1021/jm049110x
Jégou S, Cartier D, Dubessy C, Gonzalez BJ, Chatenet D, Tostivint H, Scalbert E, LePrince J, Vaudry H, Lihrmann I (2006) Localization of the urotensin II receptor in the rat central nervous system. J Comp Neurol 495(1):21–36. doi:10.1002/cne.20845
Clark SD, Nothacker HP, Wang Z, Saito Y, Leslie FM, Civelli O (2001) The urotensin II receptor is expressed in the cholinergic mesopontine tegmentum of the rat. Brain Res 923(1–2):120–127. doi:10.1016/S0006-8993(01)03208-5
MacLean MR, Alexander D, Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Morecroft I, Polland K (2000) Contractile responses to human urotensin-II in rat and human pulmonary arteries: effect of endothelial factors and chronic hypoxia in the rat. Br J Pharmacol 130(2):201–204. doi:10.1038/sj.bjp.0703314
Maguire JJ, Kuc RE, Davenport AP (2000) Orphan-receptor ligand human urotensin II: Receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br J Pharmacol 131(3):441–446. doi:10.1038/sj.bjp.0703601
Ross B, McKendy K, Giaid A (2010) Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol 298(5):R1156–R1172. doi:10.1152/ajpregu.00706.2009
Huitron-Resendiz S, Kristensen MP, Sánchez-Alavez M, Clark SD, Grupke SL, Tyler C, Suzuki C, Nothacker HP, Civelli O, Criado JR, Henriksen SJ, Leonard CS, de Lecea L (2005) Urotensin II modulates rapid eye movement sleep through activation of brainstem cholinergic neurons. J Neurosci 25(23):5465–5474. doi:10.1523/JNEUROSCI.4501-04.2005
Douglas SA, Dhanak D, Johns DG (2004) From ‘gills to pills’: urotensin-II as a regulator of mammalian cardiorenal function. Trends Pharmacol Sci 25(2):76–85. doi:10.1016/j.tips.2003.12.005
Gartlon JE, Ashmeade T, Duxon M, Hagan JJ, Jones DN (2004) Urotensin-II, a neuropeptide ligand for GPR14, induces c-fos in the rat brain. Eur J Pharmacol 493(1–3):95–98. doi:10.1016/j.ejphar.2004.04.009
Suzuki S, Wenyi Z, Hirai M, Hinokio Y, Suzuki C, Yamada T, Yoshizumi S, Suzuki M, Tanizawa Y, Matsutani A, Oka Y (2004) Genetic variations at urotensin II and urotensin II receptor genes and risk of type 2 diabetes mellitus in Japanese. Peptides 25(10):1803–1808. doi:10.1016/j.peptides.2004.03.030
Olesen J, Steiner TJ (2004) The International classification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry 75(6):808–811
Michaels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW (2010) Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 5(6):1003–1009. doi:10.2215/CJN.06870909
Cauchi M, Robertson NP (2016) CGRP and migraine. J Neurol 263(1):192–194. doi:10.1007/s00415-015-8000-4
Karsan N, Goadsby PJ (2015) Calcitonin gene-related peptide and migraine. Curr Opin Neurol 28(3):250–254. doi:10.1097/WCO.0000000000000191
Tajti J, Szok D, Majlath Z, Tuka B, Csati A, Vécsei L (2015) Migraine and neuropeptides. Neuropeptides 52:19–30. doi:10.1016/j.npep.2015.03.006
Cernuda-Morollon E, Martinez-Camblor P, Ramon C, Larrosa D, Serrano-Pertierra E, Pascual J (2014) CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache 54(6):987–995. doi:10.1111/head.12372
Vaudry H, Do Rego JC, Le Mevel JC, Chatenet D, Tostivint H, Fournier A, Tonon MC, Pelletier G, Conlon JM, Leprince J (2010) Urotensin II, From fish to human. Ann N Y Acad Sci 1200:53–66. doi:10.1111/j.1749-6632.2010.05514.x
do Rego JC, Leprince J, Scalbert E, Vaudry H, Costentin J (2008) Behavioral actions of urotensin-II. Peptides 29(5):838–844. doi:10.1016/j.peptides.2007.12.016
de Lecea L, Bourgin P (2008) Neuropeptide interactions and REM sleep: a role for Urotensin II? Peptides 29(5):845–851. doi:10.1016/j.peptides.2008.02.009
Ng LL, Loke I, O’Brien RJ, Squire IB, Davies JE (2002) Plasma urotensin in human systolic heart failure. Circulation 106(23):2877–2880. doi:10.1161/01.CIR.0000044388.19119.02
Khan SQ, Bhandari SS, Quinn P, Davies JE, Ng LL (2007) Urotensin II is raised in acute myocardial infarction and low levels predict risk of adverse clinical outcome in humans. Int J Cardiol 117(3):323–328. doi:10.1016/j.ijcard.2006.05.016
Cheung BM, Leung R, Man YB, Wong LY (2004) Plasma concentration of urotensin II is raised in hypertension. J Hypertens 22(7):1341–1344. doi:10.1097/01.hjh.0000125452.28861.f1
Tietjen GE (2009) Migraine as a systemic vasculopathy. Cephalalgia 29(9):987–96. doi:10.1111/j.1468-2982.2009.01937.x
Malik R, Winsvold B, Auffenberg E, Dichgans M, Freilinger T (2015) The migraine-stroke connection: A genetic perspective. Cephalalgia. Dec 9. 0333102415621055. [Epub ahead of print]
Tietjen EG (2007) Migraine and ischaemic heart disease and stroke: potential mechanisms and treatment implications. Cephalalgia 27(8):981–987
Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A (2009) The migraine-ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med 9(2):215–26
Cutrer FM (2010) Pathophysiology of migraine. Semin Neurol 30(2):120–130. doi:10.1055/s-0030-1249222
Goadsby PJ (2009) Pathophysiology of migraine. Neurol Clin 27(2):335–360. doi:10.1016/j.ncl.2008.11.012
Wenyi Z, Suzuki S, Hirai M, Hinokio Y, Tanizawa Y, Matsutani A, Satoh J, Oka Y (2003) Role of urotensin II gene in genetic susceptibility to Type 2 diabetes mellitus in Japanese subjects. Diabetologia 46(7):972–976
Ong KL, Wong LY, Man YB, Leung RY, Song YQ, Lam KS, Cheung BM (2006) Haplotypes in the urotensin II gene and urotensin II receptor gene are associated with insulin resistance and impaired glucose tolerance. Peptides 27(7):1659–1667
Oztuzcu S, Ulasli M, Pehlivan Y, Cevik MO, Cengiz B, Igci YZ, Okumuş S, Arslan A, Onat AM (2013) Thr21Met (T21M) but not Ser89Asn (S89N) polymorphisms of the urotensin-II (UTS-II) gene are associated with Behcet’s disease (BD). Peptides 42:97–100. doi:10.1016/j
Tobias Freilinger, Verneri Anttila, Boukje de Vries, Rainer Malik, Mikko Kallela, Gisela M Terwindt, Patricia Pozo-Rosich,Bendik Winsvold, Dale R Nyholt, Willebrordus P J van Oosterhout, Ville Artto, Unda Todt, Eija Hämäläinen, Jèssica Fernández-Morales, Mark A Louter, Mari A Kaunisto, Jean Schoenen, Olli Raitakari, Terho Lehtimäki, Marta Vila-Pueyo, Hartmut Göbel, Erich Wichmann, Cèlia Sintas, Andre G Uitterlinden, Albert Hofman, Fernando Rivadeneira, Axel Heinze, Erling Tronvik, Cornelia M van Duijn, Jaakko Kaprio, Bru Cormand, Maija Wessman, Rune R Frants, Thomas eitinger, Bertram Müller-Myhsok, John-Anker Zwart, Markus Färkkilä, Alfons Macaya, Michel Derrari, Christian Kubisch, Aarno Palotie, Martin Dichgans, Arn M J M van den Maagdenberg & International Headache Genetics Consortium (2012) Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nature Genetics 44:777–782. doi:10.1038/ng.2307
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SG and SK conceived and designed the study. SG, FŞ, EA, SE, MK, HD, AA, ET were responsible for data acquisition. SG, SK, S.KUL and AMN were in charge of data analysis and interpretation and drafted the manuscript. SG, AMN were responsible for critical revision of this manuscript. All authors approved the final version of this manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Geyik, S., Ergun, S., Kuzudişli, S. et al. Plasma urotensin-2 level and Thr21Met but not Ser89Asn polymorphisms of the urotensin-2 gene are associated with migraines. J Headache Pain 17, 36 (2016). https://doi.org/10.1186/s10194-016-0623-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-016-0623-z