Abstract
Even though Glycymeris longior is a clam widely distributed in the SW Atlantic Ocean, little is known about its biology and life history. The present study assessed the periodicity of the internal growth increments of G. longior using thin shell sections. Each internal growth increment was composed of two alternating bands: a translucent band (light-coloured when viewed with transmitted light) and an opaque band (dark-coloured). Annual formation for each pair of bands was demonstrated. The formation of the annual growth increments was synchronous among individuals. Growth was determined from live clams collected at El Sótano, Argentine Sea (age range = 29 to 69 years). According to the growth model, G. longior grows fast during the first 5 years of life and then growth becomes slower in later years; individuals reached 50% and 90% of maximum size at 5 and 13 years of age, respectively. High variability was found in shell height for the first 10 years: differences up to 5–7 mm among individuals were registered for the first 2 years of age, and up to 11 mm between the ages of 3 and 9 years. The growth performance index phi-prime (φ′) and the index of growth performance (P) of G. longior were compared with those of other Glycymeris species. Our results indicate that G. longior is a slow-growing species with a long lifespan (maximum longevity = 69 years).
Similar content being viewed by others
Introduction
Bivalve shells show periodic deposition in the form of regular external growth rings or internal banding; information about the life history of the individual and the environment in which it lived is preserved in the shells [1]. The shell is deposited in temporal cycles that can be daily, seasonal and/or annual [2]. The assessment of such temporal frequencies is a required step in ageing methods [3, 4]. Over an annual growth cycle, an annual growth increment delimited by a growth line is deposited in the shell [2]. Each growth line represents periods of growth cessation and results from seasonal variations triggered by environmental factors (e.g. temperature, salinity, food availability), the physiology of the organism (such as reproductive cycles) or an interaction between them [5].
Clams of the genus Glycymeris are infaunal bivalves distributed worldwide, from cool-temperature waters to the tropics [6]. The genus has been studied given its commercial importance: G. glycymeris is industrially exploited in the NE Atlantic (annual catches varied from 3000 to 7000 t for the period 1999–2009, [7]); G. nummaria and G. pilosa support artisanal exploitation at Mediterranean Sea [8]; a small-scale but expanding G. grayana fishery operates in New South Wales, Australia [9]. Other species are locally consumed: G. vovan and G. scripta at the E Central Atlantic [10]; G. ovata in the E Pacific [11]; Tucetona pectunculus (previously assigned to the genus Glycymeris) and G. reevei at Philippines [12]; etc. Annual growth increments were confirmed for Glycymeris species from NE Atlantic and Mediterranean Sea [e.g. 13–19]. Individual growth was modelled for G. nummaria [14], G. bimaculata [17] and G. grayana [9]. Despite being a diverse genus and worldwide distributed, there is a gap of information on the life history of glycymerids from the Southern Hemisphere.
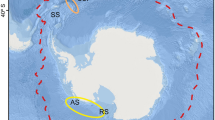
The clam Glycymeris longior (Sowerby 1832) is a common warm-temperature species of the SW Atlantic Ocean. It presents a broad continuous geographical distribution, from Espirito Santo, Brazil (20° 19′ S, 40° 20′ W) to San Matías Gulf (SMG), Argentina (41° S, 65° W) [20, 21]. Recent studies suggest a broader distribution from Pará, Brazil (00°34′ N, 47°22′ W) [22]. At the southern edge of its distribution, G. longior is an abundant species in shell assemblages from both Quaternary marine coastal and modern beaches [23]. At SMG, the species lives on the NW coast at a site called El Sótano (Fig. 1). Clams are slightly covered by the sediment (fine and medium sand) at water depths from 6 to 20 m [20]. Scientific surveys revealed that the population of El Sótano is spatially restricted to a small area. Also, it was observed that the clam shells showed several external growth lines suggesting that they were old individuals [24]. Glycymeris longior is occasionally harvested at SMG, but biological information of the population remains rudimentary which raised concerns regarding the resilience of its fringe remaining population.
Map of San Matías Gulf (SMG). a Principal hydrodynamic characteristics of SMG: circle represents the cyclonic gyre dominating the northern area of the gulf; dash line delimits the thermohaline front; arrows indicate the communication between the gulf and the continental shelf of the Argentine Sea. b Location of the sampling area at El Sótano. SW AO South West Atlantic Ocean
In the present study, under the assumption that the environmental variability affects the growth of G. longior clams, we assessed the pattern and periodicity of internal growth increments in the shells. We analysed the internal growth pattern of G. longior on shell sections to validate the periodicity of increment formation with two qualitative methods. Then we modelled the growth of individuals of G. longior and compared it with those of other species of the genus.
Materials and methods
Study area
The study was carried out at El Sótano, a site located in the NW coast of SMG, northern Patagonia, Argentina (40° 54′ S, 65° 08′ W; Fig. 1). The SMG is a semi-enclosed basin that partially communicates with the open sea through a shallow sill (60 m depth) that limits water exchange [25]. Water circulation is dominated by two eddies: one with a cyclonic gyre in the North and the other with an anticyclonic gyre in the South. During the austral spring and summer, a thermohaline front divides the water of the gulf into two masses with different oceanographic conditions. Warm and saline waters—poor in nitrate and with dinoflagellate phytoplankton—occur north of the front, whereas relatively cold and less saline waters—similar to the Patagonian shelf waters, rich in nitrates and diatom phytoplankton—occur south of the front [26, 27] (Fig. 1). The difference in temperature between both regions reaches 3 °C in summer. On the NW coast seawater temperatures range, on average, from 10.0 °C in winter (August) to 18.2 °C in summer (January) at a depth of 20 m [28]. The chlorophyll-a concentrations are characterised by two peaks, one in austral autumn (April–May) and another one in spring (September) [29]. The bottom sediment is mainly sand near the coastline and is gradually mixed with shell fragments, gravel and mud in deeper zones [30]. The predominant winds direction is NW, particularly from autumn to spring (March to November) [31]. Mean annual precipitation is 275 ± 108 mm (1980–2000) [32].
Shell sampling and processing
Between 2011 and 2014 two scientific surveys were carried out in the NW sector of the SMG to assess the epifaunal components of the benthic community (surveys design is presented as Additional file 1: Fig. S1). At each transect, divers also registered the occurrence of infaunal species. A ground of G. longior clam was registered at the south zone of El Sótano (Fig. 1). Based on this information, samples of live Glycymeris longior clams were monthly collected by SCUBA divers within an area of 4 km2 in March 2015 and from June 2015 to August 2016 (N = 1558; Additional file 2: Table S1). Divers removed sediment by hand and randomly collected buried clams. Samples were not collected in September and December 2015 as well as March 2016 due to weather conditions. In the laboratory, shell height (H, maximum shell dimension between the dorsal and the ventral margins of the shell) and shell length (L, maximum shell dimension between the anterior and posterior margins of the shell) were measured with a digital calliper to the nearest 0.01 mm. Shells were air-dried and weighted (to the nearest 0.1 g) using a digital balance.
A subsample of shells (n = 270) was randomly selected and left valves were cut along the maximum growth axis from the umbo to the ventral margin (Fig. 2a) using a Struers low-speed diamond saw (MOD13, Struers Mintom®). The internal section obtained from the first cut was ground using carborundum sandpaper (500 and 1000 grit) on the Struers LaboPol-5® rotating platform with a variable speed (100–300 rpm). The ground surface was mounted on a glass slide with cyanoacrylate and cut again to obtain a thin sections (0.5–0.8 mm). Subsequently, these thin sections were polished with carborundum papers of 1000 and 4000 grit until a uniform thickness was achieved.
Glycymeris longior. a Right valve; line represents the maximum growth axis. b Thick section of shell showing three measurements (dash lines). c Detail of ventral margin of a shell. Translucent increments (white arrows) and opaque increments (black arrows) are shown. Growth breaks in the shell surface are marked with (*). DOG: direction of growth
Validation of annual shell growth periodicity
We validated the annually formation of shell growth increment through two qualitative methods: the ‘Interval between samples method’ [4] and an adaptation of the ‘List year method’ [33]. The Interval between samples method compares samples at regular intervals. This is a well suited and commonly used method for determining the season of formation of internal bands in calcified structures by observing their evolution in marginal zones over time [34]. It is applicable to all range of ages and can be used to validate periodicity of growth increments [4]. Thin sections from samples collected in July 2015, January 2016 and July 2016 were observed under microscope (Nikon Eclipse E200). The edge of the ventral margin was categorized as opaque or translucent margin, and expressed in percentages. Association of margin category and month was determined by contingency table analysis.
The List year method is based on the assumption that the growth in all individuals for a given population is synchronic and if the date of death is known, two or more shells can be aligned to study the inter-annual variability [33]. This method consists on the identification of years with noticeable narrower (or wider) growth increments than the adjoining growth increments. Those increments are used as signal years and their presence should be noted in most sections. In addition, the number of annual growth increments among two collection dates should be consistent with the calendar years. We used shells of G. longior collected at El Sótano in June 2011 (n = 4), from the shell collection of Laboratorio de Investigaciones en Ecología Bentónica (LIEB, CIMAS). Thin sections of the shells were obtained as described before and compared with samples from July 2015 (n = 5) and January 2016 (n = 3). Digital photomicrographs of the shell portion near the ventral margin were taken under binocular microscope at 40× (Nikon Eclipse E200; Nikon camera TV Lens 0.55 × DS). Microphotographs were assembled precisely to provide a detailed panorama of the shell. In each photomontage, the year before the collection date was assigned to the foremost growth increment (2010, 2014 or 2015). Subsequently, each year was assigned by counting backwards from the last increment. Considering that analysed individuals were older than 30 years (see Additional file 3: Fig. S2) the method was applied to the last 10 years (2005–2016). Thin sections were then visually compared to confirm that each increment was assigned to the correct year of formation.
Growth
Nineteen thin shell sections were photographed under the microscope (Nikon Eclipse E200; Nikon camera TV Lens 0.55× DS) and photomontages (containing 40 photos approximately) were created with Photoshop® software. Each photomontage provided a complete view of the entire shell section. We measured the distance between the beak in the umbo and the end of each annual growth increment in the outer shell surface (Fig. 2b). Repeated measurements of shell heights for each individual were obtained throughout the ontogeny using the ImageJ software (available as freeware at https://imagej.nih.gov/ij/).
Individual growth profiles were built. Growth parameters were estimated through fitting a variation of the von Bertalanffy Growth Model (VBGM) [35] to each profile using non-linear mixed-effects models [36]. Growth parameters of each individual were expressed as the sum of a population mean parameter (“fixed effect”) and an individual deviation (“random effect”) [37].
Growth parameters estimation through mixed-effect models allows the incorporation of different growth variability sources to the correlation analysis for grouped data: (i) between individuals (random effects), and (ii) within-individuals (random error). The model used for the individual growth increments, was given by the equation
Hij represents the height at age j of the individual i, H∞, and K represent the average population parameters of the VBGM (fixed effects); h∞i, and ki are the random effects of population parameters of the VBGM of the individual, assumed to be normally distributed, with zero mean and variance–covariance matrix σ2Di of a 3 × 3 dimension with diagonal elements var(h∞) and var(k) and off-diagonal elements given by cov(h∞, k). The term εij is the height variability associated with the age j, and represents the process stochasticity (random error) assumed to be normally distributed with zero mean and variance–covariance matrix. The significance of each population parameter (fixed effect) was assessed by conditional F-tests and the significance of individual variability of each of the model parameters by comparing the AIC [36]. The dependence of sequential observations within-individuals was evaluated and parameterized using a first-order auto-regressive function (AR1). The variance of εij was parameterized as a power function of the j (age in years)
The growth performance index phi-prime (φ′) was estimated to compare growth of G. longior with other species of the genus. The index was calculated by the equation φ′ = log K + 2logH∞ [38]. Growth parameters K and H∞ from all species were used to calculate the index of growth performance \(P = \log \left( {k \times H_{\infty }^{3} } \right)\) [39]. The logarithmic transformations of both variables were plotted in an ‘auximetric grid’ which graphically represents differences among species [39].
Results
Analysis of internal growth pattern
The internal shell growth increments were composed of two alternating increments: a translucent increment (light-coloured band) and an opaque increment (dark-coloured band) (Fig. 2b). The translucent increment was a relatively narrow increment that appears translucent in shell sections if viewed in transmitted light. The wide opaque increment was opaque in transmitted light, but dark brown in hand sample or if viewed in reflected light. The growth increment widths exponentially declined towards the ventral margin (Fig. 2b). A growth break was visible in the shell surface between translucent and opaque increments (Fig. 2b). Both internal growth increments were visible in the outer and the inner shell layers (Fig. 2b). The hinge plate was pale in almost all specimens.
Validation of annual shell growth periodicity
Annual increment formation in Glycymeris longior was confirmed by growth increments formed between 2011 and 2015/2016. A total of four growth increments (i.e. combination of an opaque and a translucent increment) were counted between 2011 and 2015, and five increments between 2011 and 2016. We found three distinct signal years: wide increments were registered in 2007 and 2010, whilst narrow increments were found in 2013 (Fig. 3). No missing annual growth increments were identified. The margin category was associated with sampling month (p = 0.0002).
Three ventral margins photographed on thick sections of shells of Glycymeris longior from El Sótano, San Matías Gulf, collected in a January 2016, b July 2015 and c June 2011. Wide annual increments were registered in 2007 and 2010 (dark grey bands; common for all images); narrow increments were found in 2013 (in a and b). Scale bar: 250 μm
The estimated ages of the clams used in this study ranged between 29 and 69 years, but the samples were mainly composed of 35–39 years old individuals (Additional file 3: Fig. S2).
Growth
Growth pattern was similar for all individuals. Shell height (H) as a function of time (age) showed fast growth during early life and a slower growth for later years. However, high variability was found in shell height for the first ten years: differences up to 5–7 mm among individuals were registered for the first 2 years of age; differences up to 11 mm between the ages of 3 and 9 years. The von Bertalanffy model provided a representation of the individual growth profiles (Fig. 4). We selected the full model with modelling of the variance as a power function of the expected growth increment. The residuals were serially correlated (estimated autocorrelation equal to 0.64). For this model H∞ was 39.40 mm (SE = 0.34) and k was 0.18 (SE = 0.006). According to the model, individuals reached 80% of maximum H at 9 years and 90% at 13 years of age (Fig. 4).
Individual growth profiles of Glycymeris longior clams (n = 19). Observed shell heigth (dots, n = 706) and fitted values (line) for the mixed-effects version of the von Bertalanffy model. Variance was parameterized as a power function of the expected shell heigth. The residuals were serially correlated
The φ′ value and the P value calculated for G. longior were 2.45 and 4.04, respectively. Table 1 presents growth parameters for Glycymeris clams. Figure 5 presents the auximetric grid (Additional file 4: Table S2).
Auximetric grid for comparison of the index of growth performance (P) among Glycymeris species. Savina [44] reported four different growth parameters for the G. glycymeris populations at Bay of Saint-Brieuc, France. Gray lines connecting P values indicate the same growth performance
Discussion
Age estimation in bivalves is usually based on records of environmental changes represented in their shells [1, 2]. A successful interpretation of growth increments needs an accurate determination of periodicity of their formation [3, 40]. The methods applied here confirmed an annual periodicity in the deposition of the G. longior growth increments. In an annual cycle, G. longior shells exhibit an alternation of two repeating growth increments: a relatively narrow translucent increment and a wide opaque increment. The growth pattern suggests that the species growths during the whole year in two different growing seasons, which might be related with the environmental seasonality of the area. Other glycymerids species also show annual growth patterns (G. bimaculata [17]; G. glycymeris [13, 16]; G. nummaria [14]; G. vanhengstumi [41]. Some species even show such pattern in their fossil material (G. planicostalis) [42].
Seawater temperature is often the main driver and influences growth direct and/or indirectly. Bio-mineralization rate occurs within a thermal threshold and lower or higher temperatures lead to a slowdown or a cessation of growth [5]. This results in a discontinuity in the shell and the formation of annual growth lines. Many glycymerids present a minimum thermal threshold for shell accretion. As an example, G. glycymeris slows down or ceases shell growth below 13 °C [16]. Similar thermal limitations were found for G. bimaculata; no shell material is deposited when seawater temperatures dropped below ~ 12 to 14 °C at Eastern Adriatic Sea [18]. Similarly, the timing of growth line formation in G. nummaria [14] coincides with minimum seawater temperature. In the case of G. bimaculata, it was demonstrated that the deposition occurs in the early spring (> 14 °C) [17]. Our results suggest that G. longior has an extended growing season. Since seawater temperature at El Sótano oscillates between 10.0 and 18.2 °C [28], it is plausible that G. longior slows down growth at lower temperatures. In addition, growth breaks are observed in the shell surface at the end of the translucent increment (Fig. 2), presumably due to lower temperatures. Even though the alternating coloration of the increments in G. longior suggests that annual accretion takes place during contrasting environmental regimes, however the timing of the formation of each increment cannot be precisely estimated using our results. Further studies are needed to establish relationships between environmental parameters and shell growth.
Individual growth curves of G. longior described a fast growth for the first years of life. Individuals reached 50% and 90% of asymptotic size at 5 and 13 years of age, respectively. This growth pattern, also described for G. bimaculata and G. nummaria [14, 17], might provide an advantage in competition for space [43]. Our results demonstrated synchronic growth among individuals of G. longior for the population of SMG. Clams analyzed in our study were collected in a small area thus were exposed to the same environmental forcing during their growth. However, variations in the growth rates during the first years of life resulted in different maximum heights among individuals. Height differences reach up to 11 mm between ages of 3- and 9 years and up to 5–7 mm in younger ages. The differences among individual growth rates might be explained by density gradient or environmental differences at a micro-spatial scale. Glycymerids are slow mobile burrowers [6]; they can move and relocate in the vertical substrata, but the unspecialized burrowing is inefficient and limits the horizontal motility. Differences in growth rate among individuals of the same populations have been also reported for other glycymerids [14, 17] with differences up to almost 15 mm in size among individuals of the same age. Further studies are needed to elucidate specific mechanisms behind this variability in individual growth rates.
The Glycymeris species showed similar growth performance values (P) and the calculated φ′ displayed a narrow range (2.45–2.77). The auxometric grid allows the definition of taxa by means of their growth parameters; it has been suggested that such parameters represent the energetics of a given habitat or niche because is directly associated to growth performance and hence metabolism and food consumption [38, 39]. These results are not surprising given that the Glycymerididae family has retained the same simple shell morphology which suggests a conserved ecology [6]. The three species compared in the present study live in similar environments: loose, unstable sand and gravel; strong hydrodynamics; similar temperature regime [9, 44].
Growth in Glycymeris genus seems to be driven by a complex combination of environmental factors, especially for populations that occur in shallow coasts with highly variable conditions [45]. Growth of G. pilosa was positively associated with precipitation which increases coastal runoff and nutrient discharge and results in enhancement of primary production [45]. Growth of G. vanhengstumi from NE Atlantic depends on the surface primary production conditioned by the upward transport of the nutrients fertilizing the photic zone, which is driven by the late-winter cooling of the sea surface and ocean mixing [41]. The precipitation regime in the SMG is typical of a semiarid region (275 mm year−1) [32]. The effect of nutrient discharge in this region is predominantly driven by the NW winds [46]. In Patagonia, dust can be considered a significant source of NO3− and Si(OH)4 that benefits primary productivity of oligotrophic coastal waters in the SW Atlantic Ocean [46]. The combination of optimal temperatures and food availability during the pulses of primary production might affect growth for G. longior at SMG. There are no studies regarding the prey items of G. longior but as at El Sótano it lives buried on the first centimetres of the sediment, its diet might be similar to other species from the genus. The main diet items of G. glycymeris in Southern Brittany, France [47] and G. nummaria in the Adriatic Sea [48] are phytoplankton and bacteria associated to detrital materials on sediments.
It is unlikely that the reproductive cycle affects shell growth in G. longior. The reproductive cycle of G. longior is continuous and peaks twice in a year, in autumn and in late spring (Orrego, personal communication). This reproductive feature is consistent with an extended growing season. The El Sótano population has no resting period, thus a new gametogenetic cycle begins immediately after spawning events.
Glycymeris longior longevity (up to 69 years) is similar to the glycymerids from mid-latitudes of the Northern Hemisphere. Similar longevities were reported for G. glycymeris reported from the Irish Sea (68 years) [15], for G. glycymeris from the NE Atlantic (70 years) [49] and for G. pilosa from the Adriatic Sea (69 years) [45]. Glycymeris longior is part of the group of the most long-lived species of Argentine Sea. The life-span together with the interannual variability makes Glycymeris longior a potential species to be used as environmental proxy archive for the SW Atlantic Ocean. These aspects are currently under study.
It has been stated that the dynamic of fringe populations (near the distribution limits of the species) fluctuates due to the factors that determine the geographic distributional limit of the species [50]. Age estimation of G. longior collected at El Sótano were restricted to few and old year classes. From this observation two hypotheses arise:
- i.
The population of El Sotano is isolated and supported by sporadic pulses of recruitment. At the limit of its geographical distribution, a population might have lower opportunities for a successful recruitment due to environmental constrains. However, this effect can be buffered through the longevity of the individuals. Periodic successful recruitments that result in one or a few year-classes population have already been observed in other bivalve species at SMG. The clam Amiantis purpurata is a warm-temperate water species living at SMG as a fringe population at the southern edge of its geographical distribution. Only two historical successful recruitments at SMG occurred in 1979 and in 1980 [51]. Our results suggest that A. purpurata and G. longior populations recruited in the same years. A geographical coherence in settlement among populations located within the same region is generally considered as an indication of external forcing at a large-geographic scale [52].
- ii.
Recruitment takes place in other patches. A spatial segregation among age classes is plausible given that bivalve populations are often structured into metapopulations [53]. According to this hypothesis, in the neighboring area there would be other patches with different age composition and interconnected by larval dispersion. Indeed, a small population constituted by young clams (≤ 30 years) was documented inside the San Antonio Bay [54]. However, this population disappeared; at present, only small-empty shells remain buried in life positioning (Doldan, unpublished data). Further exploratory surveys—specific for infaunal species- are needed.
Recruitment is a complex process determined by numerous factors operating and interacting on multiple time and spatial scales in several environments [55,56,57]. Such context implies considerations at wide temporal scales. In paleontological studies, populations of G. longior have been found inside San Antonio Bay (S. Bayer, personal communication). Also, shells deposits on the NW of the SMG intertidal zone suggest that the species was abundant during the mid-Holocene, when the sea level rose and a temperature increased. The subsequent post-glacial cooling period generated a northward displacement of the geographical distribution of several warm species. However, some warm-temperate water living species, such as Arenicola brasiliensis (Polychaeta) [58], Hippocampus sp. (Pisces) [59] and A. purpurata [60] remained geographically restricted to the San Antonio Bay. These relictual populations survived probably due to the summer warm temperatures (18.2 °C in average) [28] that allow successful reproduction and survival of critical life stages [60]. The population of G. longior might be also a relic which persistence relies on sporadic recruitment pulses that succeed only when specific hydrological and thermal conditions concur. Geomorphological characteristics of San Antonio Bay—low slope of the beach and high tidal amplitude − might favour higher summer temperatures that are suitable for the species. Restricted water circulation in the NW of the SMG is verified in the retention of larval patches of bivalves of the area such as A. purpurata [51] and Ostrea puelchana [61]. This could also be the case for G. longior.
Our results lead to new questions about the factors that determine the dynamics of G. longior populations (e.g. environmental factors, density dependence, predation upon the reproductive stock, human disturbance) and the temporal and spatial scales at which they operate. Even when harvest is occasional, a clam population with sporadic pulses of recruitment and slow individual growth brings up concerns regarding its management and conservation. Future studies that include reproduction, diet, larval recognition, and benthic surveys to describe spatial distribution and density gradients are needed to understand the dynamics of populations of G. longior and to report their conservation and management.
Conclusion
The present study successfully estimated age and growth of Glycymeris longior. We assessed the periodicity of the internal growth increments in thin sections of shell. Each growth increment was composed of two alternating bands and an annual formation for each pair of bands was demonstrated. According to the growth model, G. longior grows fast during the first 5 years of life and then growth slows down in the later years (maximum longevity of 69 years). The growth performance index phi-prime (φ′) and the index of growth performance (P) of G. longior showed similarities with those of other Glycymeris species. This result is in concordance with the simple shell morphology and conserved ecology of the Glycymerididae family. The individuals analyzed in this study belong to the population at the southern limit of distribution of the species; it is plausible that the environmental factors that determine the geographic range might also affect the growth performance at this latitude. Future studies should assess growth patterns throughout the distribution range in order to test heterogeneity among populations. The slow growth and great longevity suggest that G. longior is vulnerable, an aspect that must be taken into account if a fishery starts operating.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rhoads DC, Lutz RA. Skeletal growth of aquatic organisms: biological records of environmental change. New York: Plenum Press; 1980.
Richardson C. Molluscs as archives of environmental change. Oceanogr Mar Biol Annu Rev. 2001;39:103–64.
Beamish RJ, McFarlane GA. The forgotten requirement for age validation in fisheries biology. Trans Am Fish Soc. 1983;112(6):735–43.
Campana SE. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol. 2001;59:197–242.
Schöne BR. The curse of physiology—challenges and opportunities in the interpretation of geochemical data from mollusk shells. Geo Mar Lett. 2008;28:269–85.
Thomas RDK. Functional morphology, ecology, and evolutionary conservatism in the Glycymerididae (Bivalvia). Palaeontology. 1975;18:217–54.
FAO. http://www.fao.org. Accessed 1 Nov 2019.
Poutiers JM. Bivalves (Acéphales, Lamellibranches, Péléccypodes). In: Fisher W, Bauchot ML, Scheider M, editors. Fiches FAO d´identification des espèces pour les besoins de la pêche 37. Volume 1. Végétaux et Invertébrés. Rome: FAO; 1987. p. 369–512.
Beaver PE, Bucher DJ, Joannes-Boyau R. Growth patterns of three bivalve species targeted by the Ocean Cockle Fishery, southern New South Wales: Eucrassatella kingicola (Lamarck, 1805); Glycymeris grayana (Dunker, 1857); and Callista (Notocallista) kingii (Gray, 1827). Molluscan Res. 2017;37:104–12.
Abbot RT. Bivalves. In: Fischer W, Bianchi G, Scott WB, editors. FAO species identification sheets for fishery purposes Eastern Central Atlantic (Fishing Areas 34, 47 in part). Rome: FAO; 1982. p. 1–17.
Arguelles J, Taipe A, Srmiento M, Valdez R. Prospección del recurso mejillón Glycymeris ovata en el área del callao (12–13 Mayo 2013). Instituto del Mar del Perú. 2014.
Poutiers JM. Bivalves of the world. Annotaed list of marine and brackish water species of interest to fisheries. FAO Catalogue. 1992 (Unpublished).
Berthou P, Blanchard M, Noël P, Vergnaud-Grazzini C. Stable isotope analysis of shells for age determination in four bivalve species from the Normand-Breton Gulf (western English Channel). International Council for the Exploration of the Sea Shellfish Committed, report. 1986.
Peharda M, Crnčević M, Bušelić I, Richardson CA, Ezgeta-Balić D. Growth and longevity of Glycymeris nummaria (Linnaeus, 1758) from the eastern Adriatic, Croatia. J Shellfish Res. 2012;31:947–50.
Brocas WM, Reynolds DJ, Butler PG, Richardson CA, Scourse JD, Ridgway ID, Ramsay K. The dog cockle, Glycymeris glycymeris (L.), a new annually-resolved sclerochronological archive for the Irish Sea. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;373:133–40.
Royer C, Thébault J, Chauvaud L, Olivier F. Structural analysis and paleoenvironmental potential of dog cockle shells (Glycymeris glycymeris) in Brittany, northwest France. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;373:123–32.
Bušelić I, Peharda M, Reynolds DJ, Butler PG, González AR, Ezgeta-Balić D, Vilibić I, Grbec B, Hollyman P, Richardson CA. Glycymeris bimaculata (Poli, 1795)—a new sclerochronological archive for the Mediterranean? J Sea Res. 2015;95:139–48.
Peharda M, Thébault J, Markulin K, Schöne BR, Janeković I, Chauvaud L. Contrasting shell growth strategies in two Mediterranean bivalves revealed by oxygen-isotope ratio geochemistry: the case of Pecten jacobaeus and Glycymeris pilosa. Chem Geol. 2017. https://doi.org/10.1016/j.chemgeo.2017.09.029.
Reynolds DJ, Hall IR, Slater SM, Scourse JD, Halloran PR, Sayer MDJ. Reconstructing past seasonal to multicentennial-scale variability in the NE atlantic ocean using the long-lived marine bivalve mollusk Glycymeris glycymeris. Paleoceanography. 2017;32:1153–73.
Scarabino V. Moluscos del golfo San Matías (provincia de Río Negro, República Argentina). Inventario y claves para su identificación. Comunicaciones de la Sociedad Malacológica del Uruguay. 1977;4:177–285.
Zelaya DG. Marine bivalves from the Argentine coast and continental shelf: species diversity and assessment of the historical knowledge. Am Malacol Bull. 2015;33:245–62.
Rocha VP, Matthews Cascon H. The family glycymerididae (Mollusca: Bivalvia) from North and Northeast Brazil. Arquivos de Ciências do. 2014;47(2):64–71.
Bayer MS, Gordillo S. A new Pleistocene species of Glycymeris (Bivalvia, Glycymerididae) from northern Patagonia, Argentina. Ameghiniana. 2013;50(2):265–9.
Doldan MS. Patrones y procesos de la dinámica poblacional de la ostra puelche, Ostrea puelchana, (D’ Orbigny, 1842). PhD thesis. Universidad de Buenos Aires, Argentina. 2013.
Rivas AL, Beier EJ. Temperature and salinity fields in the North Patagonian gulfs. Oceanol Acta. 1990;13:15–20.
Piola AR, Scasso LM. Circulación en el golfo San Matías. Geoacta. 1988;15:33–51.
Gagliardini DA, Rivas AL. Environmental characteristics of San Matías Gulf obtained from LANDSAT-TM and ETM + data. Gayana. 2004;68:186–93.
Williams GN, Zaidman PC, Glembocki NG, Narvarte MA, González RA, Esteves JL, Gagliardini DA. Comparison between remotely-sensed sea-surface temperature (AVHRR) and in situ records in San Matías Gulf (Patagonia, Argentina). LAJAR. 2014;2:192–203.
Williams GN, Dogliotti AI, Zaidman P, Solis M, Narvarte MA, Gonzalez RC, Esteves JL, Gagliardini DA. Assessment of remotely-sensed sea-surface temperature and chlorophyll-a concentration in San Matías Gulf (Patagonia, Argentina). Cont Shelf Res. 2013;52:159–71.
Morsan EM, Pappalardo P, Doldan MS. Growth compensation as a regulatory mechanism of purple clam Amiantis purpurata population dynamics in Patagonia. Mar Ecol Prog Ser. 2011;443:207–16.
Genchi SA, Carbone ME, Piccolo MC, Perillo ME. Déficit hídrico en San Antonio Oeste, Argentina. Revista de Climatología. 2010;10:29–43.
Servicio Meteorológico Nacional http://www.smn.gob.ar/.
Yamaguchi DK. A simple method for cross-dating increment cores from living trees. Can J For Res. 1991;21(3):414–6.
Panfili, J, Morales-Nin, B. Semi-direct validation. In: Panfili J, Pontual H, Troadec H, Wright PJ, eds. Manual of fish sclerochronology. Ifremer-IRD coedition, Brest (France). 2002. p. 129–34.
Quinn TJ, Deriso RB. Quantitative fish dynamics. Oxford: Oxford University Press; 1999.
Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer-Verlag; 2002 (Corrected third printing).
Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–87.
Munro JL, Pauly D. A simple method for comparing the growth of fishes and invertebrates. Fishbyte. 1983;1:5–6.
Pauly D. Gill size and temperature as governing factors in fish growth: a generalization of von Bertalanffy’s growth formula. 1979.
Penttila J, Dery LM. Age determination methods for Northwest Atlantic species. NOAA Technical Report. NMFS. 1988;72:129–32.
Németh A, Kern Z. Sclerochronological study of a Glycymeris vangentsumi population from the Madeira Islands. Front Earth Sci. 2018;6:76.
Walliser EO, Schöne BR, Tütken T, Zirkel J, Grimm KI, Pross J. The bivalve Glycymeris planicostalis as a high-resolution paleoclimate archive for the Rupelian (Early Oligocene) of central Europe. Clim Past. 2015;11:653–68.
Arendt JD. Adaptive intrinsic growth rates: an integration across taxa. Q Rev Biol. 1997;72:149–77.
Savina M. Modélisation écologique des populations de palourdes roses (Paphia rhomboïdes) et d’amandes de mer (Glycymeris glycymeris) en Manche. Ph.D. Thesis, Université d’Aix-Marseille II. 2004.
Peharda M, Black BA, Purroy A, Mihanović H. The bivalve Glycymeris pilosa as a multidecadal environmental archive for the Adriatic and Mediterranean Seas. Mar Environ Res. 2016;119:79–87.
Paparazzo FE, Crespi-Abril AC, Gonçalves RJ, Barbieri ES, Gracia Villalobos LL, Solís ME, Soria G. Patagonian dust as a source of macronutrients in the Southwest Atlantic Ocean. Oceanography. 2018;31(4):33–9.
Galap C, Netchitaı̈lo P, Leboulenger F, Grillot J-P. Variations of fatty acid contents in selected tissues of the female dog cockle (Glycymeris glycymeris L., Mollusca, Bivalvia) during the annual cycle. Comp Biochem Physiol A: Mol Integr Physiol. 1999;122:241–54.
Najdek M, Ezgeta-Balić D, Blažina M, Crnčević M, Peharda M. Potential food sources of Glycymeris nummaria (Mollusca: Bivalvia) during the annual cycle indicated by fatty acid analysis. Sci Mar. 2016;80:123–9.
Featherstone AM, Butler PG, Peharda M, Chauvaud L, Thébault J. Influence of riverine input on the growth of Glycymeris glycymeris in the Bay of Brest, North-West France. PLoS ONE. 2017;12:e0189782.
Naidu KS, Anderson JT. Aspects of scallop recruitment on St. Pierre Bank in relation to oceanography and implications for resource management. CAFSAC Res. Doc. 84/29. 1984. 15 pp.
Morsan EM, Orensanz J. Age structure and growth in an unusual population of purple clams, Amiantis purpuratus (Lamarck, 1818) (Bivalvia; Veneridae), from Argentine Patagonia. J Shellfish Res. 2004;2004(23):73–80.
Arnold WS, Marelli DC, Bray CP, Harrison MM. Recruitment of bay scallops Argopecten irradians in Floridan Gulf of Mexico waters: scales of coherence. Mar Ecol Prog Ser. 1998;170:143–57.
Orensanz JL, Parma AM, Smith SJ. Dynamics, assessment, and management of exploited natural scallop populations. Developments in aquaculture and fisheries science. New York: Elsevier; 2016. p. 611–95.
Ituarte CF. Sobre la sexualidad de Glycymeris longior (Sowerby) (Mollusca Pelecypoda). Neotropica. 1979;25:161–5.
Nakaoka M. Yearly variation in recruitment and its effect on population dynamics in Yoldia notabilis (Mollusca: Bivalvia), analyzed using projection matrix model. Res Popul Ecol. 1993;35:199–213.
Eckman JE. Closing the larval loop: linking larval ecology to the population dynamics of marine benthic invertebrates. J Exp Mar Biol Ecol. 1996;200:207–37.
Roughgarden J, Gaines S, Possingham H. Recruitment dynamics in complex life cycles. Science. 1988;241:1460–6.
Amor A. The larval development of Arenicola brasiliensis Nonato Polychaeta, Arenicolidae. Physis. 1981;39:79–83.
Piacentino GL, Luzzatto DC. Hippocampus patagonicus sp. nov., nuevo caballito de mar para la Argentina (Pisces, Syngnathiformes). Revista del Museo Argentino de Ciencias Naturales. 2004;6:339–49.
Morsan EM, Kroeck MA. Reproductive cycle of purple clam, Amiantis purpurata (Bivalvia: Veneridae) in northern Patagonia (Argentina). J Mar Biol Assoc UK. 2005;85:367–73.
Pascual MS, Zampatti EA, Iribarne OO. Population structure and demography of the puelche oyster (Ostrea puelchana, D’Orbigny, 1841) grounds in Northern Patagonia, Argentina. J Shellfish Res. 2001;20:1003–10.
Acknowledgements
We thank S. Acosta, J. Lopez and N. Dieu for the collaboration in sampling collection, and M.E. Orrego and F.E. Surdo for helping with the processing of samples. We gratefully thank Konrad Górski (UCSC Chile) for his constructive comments that helped to improve the manuscript. This research is part of the B.Sc. dissertation of LHG.
Funding
This research was supported by projects M025 and M026 from Universidad Nacional del Comahue.
Author information
Authors and Affiliations
Contributions
LHG: participated in the sample processing and data acquisition, analysed and interpreted data, prepared illustrations for the article, and drafted, wrote, prepared and edited the manuscript. MSD: design of the work, data acquisition, analysed and interpreted data, drafted the manuscript and was a major contributor in writing and correcting the manuscript. PCZ analysed and interpreted data, substantively revised the manuscript. EMM design of the work, participated in the sample processing, drafted and substantively revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Fig. S1.
NW sector of the San Matías Gulf with the location of the 98 transects (grey points) of the scientific surveys carried out in 2011–2014. Transects (1 m-wide and 50 m-long) were separated by 0.5° latitude (0.5 nautical miles ≈ 925 m) and by 0.5° longitude (≈ 720 m). Black dots: transects where Glycymeris longior clams were registered. The size of the dot is a qualitative measure of the clam density.
Additional file 2: Table S1.
Samples of Glycymeris longior collected between 2015 and 2016 at El Sótano.
Additional file 3: Fig. S2.
Glycymeris longior population structure at El Sótano, San Matías Gulf. A. Size frequency distribution (n = 1558). B. Age frequency distribution (n = 270).
Additional file 4: Table S2.
Morphometric data of analysed G. longior individuals used to model growth.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gimenez, L.H., Doldan, M., Zaidman, P.C. et al. Age and growth of Glycymeris longior (Sowerby, 1832) clam at the southern edge of its distribution (Argentine Sea). Helgol Mar Res 74, 2 (2020). https://doi.org/10.1186/s10152-020-0534-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10152-020-0534-x