Abstract
Introduction
Our objective was to investigate whether a lack of frizzled-related protein B (FrzB), an extracellular antagonist of the Wnt signaling pathways, could enhance cartilage degradation by facilitating the expression, release and activation of matrix metalloproteinases (MMPs) by chondrocytes in response to tissue-damaging stimuli.
Methods
Cartilage explants from FrzB−/− and wild-type mice were challenged by excessive dynamic compression (0.5 Hz and 1 MPa for 6 hours). Load-induced glycosaminoglycan (GAG) release and MMP enzymatic activity were assessed. Interleukin-1β (IL-1β) (10, 100 and 1000 pg/mL for 24 hours) was used to stimulate primary cultures of articular chondrocytes from FrzB−/− and wild-type mice. The expression and release of MMP-3 and −13 were determined by RT-PCR, western blot and ELISA. The accumulation of β-catenin was assessed by RT-PCR and western blot.
Results
Cartilage degradation, as revealed by a significant increase in GAG release (2.8-fold, P = 0.014) and MMP activity (4.5-fold, P = 0.014) by explants, was induced by an excessive load. Load-induced MMP activity appeared to be enhanced in FrzB−/− cartilage explants compared to wild-type (P = 0.17). IL-1β dose-dependently induced Mmp-13 and −3 gene expression and protein release by cultured chondrocytes. IL-1β-mediated increase in MMP-13 and −3 was slightly enhanced in FrzB−/− chondrocytes compared to wild-type (P = 0.05 and P = 0.10 at gene level, P = 0.17 and P = 0.10 at protein level, respectively). Analysis of Ctnn1b and Lef1 gene expression and β-catenin accumulation at protein level suggests that the enhanced catabolic response of FrzB−/− chondrocytes to IL-1β and load may be associated with an over-stimulation of the canonical Wnt/β-catenin pathway.
Conclusions
Our results suggest that FrzB may have a protective role on cartilage degradation and MMP induction in mouse chondrocytes by attenuating deleterious effects of the activation of the canonical Wnt/β-catenin pathway.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a common multifactorial joint disease, with ageing and excessive loading as important risk factors. Although the pathophysiology of OA involves cartilage, bone and the synovial tissue, the main feature of OA remains the progressive degradation of articular cartilage. Progressive joint destruction in OA has been associated with overactivation of Wnt signaling in numerous studies [1–6]. The Wnt signaling pathway is a potent regulator of bone and cartilage homeostasis and also has a role in human joint diseases [7, 8]. The canonical Wnt pathway is initiated by binding of Wnt ligands to frizzled receptors and co-receptors, low-density lipoprotein receptors (LRP-5/6), which leads to intracellular β-catenin stabilization and accumulation, nuclear translocation, interaction with transcription factors, T-cell factor and lymphoid enhancer binding factor (LEF), and, finally, activation of target genes. The noncanonical Wnt pathways involve specific ligands and are independent of β-catenin and LRPs. Wnt ligands such as Wnt-7b, Wnt-16 and the Wnt target gene Wnt-1 inducible signaling pathway protein 1 (Wisp-1) were found upregulated in OA cartilage [1–3]. In addition, β-catenin, the co-receptor LRP-5 and the transcription factor LEF-1 were found overexpressed [4–6]. Frizzled-related protein B (FrzB) is an extracellular antagonist of the Wnt signaling pathway, also called secreted Frizzled-related protein 3 (sFRP-3). As FrzB can bind Wnts in the extracellular space and prevent ligand–receptor interaction, the protein can be considered an antagonist of both canonical and noncanonical signaling. Two single-nucleotide polymorphisms in FRZB, which are loss-of-function mutations, were associated with an increased risk of OA [9–13]. However, this association was challenged by recent studies [14–17], so the potential role of FrzB in OA is still controversial.
Studies in mouse models of OA corroborated the association between cartilage degradation and an overactivation of Wnt signaling. In particular, Wisp-1 was found upregulated in two models of OA; moreover, local overexpression of Wisp-1 enhanced cartilage damage [3]. Transgenic mice that produced activated-β-catenin in adult chondrocytes developed an OA-like phenotype upon ageing [4]. FrzB−/− knockout mice did not develop spontaneous OA, but the deletion of FrzB increased cartilage loss in three different models of arthritis [18]. FrzB may thus have a protective role on OA progression. How FrzB can influence OA process remains largely unclear, but various hypotheses have been suggested [4, 18–22]. The canonical Wnt pathway is crucial for proper chondrocyte differentiation in early developmental processes to control chondrogenesis and, later, to regulate hypertrophic maturation. Abnormal Wnt signaling in the absence of FrzB could cause aberrant skeletal morphogenesis, and variations in human hip shape have been associated with the abovementioned FrzB polymorphisms [19]. This signaling could contribute to the development of OA by increasing the biomechanical burden on the articular cartilage [19, 23]. In addition, OA is also characterized by hypertrophy-like changes in chondrocytes, which could be enhanced by an overactivation of Wnt signaling in absence of FrzB [24].
In cultured chondrocytes, Wnt-3a – a commonly used Wnt ligand that triggers β-catenin signaling – increased the expression of matrix metalloproteinase (MMP)-3 and MMP-13, and MMP-2 and MMP-9 enzymatic activities [25–27]. In transgenic mice, activated β-catenin increases the expression of MMP-2, MMP-3, MMP-7, MMP-9 and MMP-13 [4, 28]. Similarly, downregulation of LRP-5 decreased the expression of MMP-7, MMP-9, MMP-13 and MMP-14 [5, 6]. The transcription complex formed by activated-β-catenin and Lef-1 has been shown to strongly bind MMP-9, MMP-13 and MMP-14 promoters, especially [5, 29].
We focused on the hypothesis that the absence of FrzB could favor OA-like catabolic processes in cartilage by increasing the activation of the Wnt signaling pathway. We therefore studied cartilage degradation in FrzB KO mice, after biomechanical loading or cytokine treatment.
Methods
Animals
All experiments were made on explants or primary chondrocytes extracted from 3-day-old to 6-day-old newborn litters from FrzB−/− or wild-type mice [18]. All procedures were in accordance with the European Directive N886/609 and were performed according to the protocols approved by French and European ethics committees for animal use and care (Comité Régional d’Ethique en Expérimentation Animale N°3 de la région Ile de France).
Compression of costal cartilage explants
The procedure for compressive loading of mouse costal cartilage explants was as described previously [30]. Briefly, explants were harvested from rib cages of 4-day-old to 6-day-old newborn mice. Samples were cleaned, divided into segments, pooled and weighed for further normalization; each sample consisted of around 30 to 40 mg costal cartilage. The explants were allowed to rest for about 20 hours in 3 ml serum-free medium (Dulbecco’s modified Eagle’s medium supplemented with 0.1% bovine serum albumin and 30 mM Hepes). They were washed before they underwent 6-hour dynamic compression in 1.5 ml of the same fresh medium (sinusoidal wave-form 0 to 1 MPa at 0.5 Hz) by the Biopress system (Flexercell International, Dunn Labortechnick GmbH, Asbach, Germany). Control explants were kept in unloaded conditions. After the application of the mechanical regimen, supernatants and cartilage explants were collected and stored immediately at −80°C. Our results are expressed as fold-induction versus the uncompressed explant controls.
Primary culture of mouse articular chondrocytes
Primary chondrocytes were isolated from articular cartilage of 4-day-old to 6-day-old newborn mice as described previously [31]. After 1 week of expansion, the cells were placed in serum-free conditions for 24 hours (0.1% bovine serum albumin; Sigma Aldrich, Saint-Quentin Fallavier, France) and then treated for 24 hours in serum-free medium supplemented with 0, 10, 100 or 1,000 pg/ml interleukin (IL)-1β (PeproTech, Tebu-Bio, Le Perray-en-Yvelines, France). Our results are expressed as fold-induction versus the nontreated controls.
RNA extraction, reverse transcription and real-time polymerase chain reaction
RNA was extracted from chondrocytes cultured with and without IL-1β by use of the RNeasy minikit (Qiagen, Courtaboeuf, France); for the RNA extraction from compressed and uncompressed cartilage explants, Proteinase K (Qiagen) was first added to remove proteins as suggested for tissue samples by the manufacturer. Reverse transcription was performed on 1 μg RNA for monolayer cultured cells and on 100 to 200 ng RNA for explants using the Omniscript kit (Qiagen). Relative quantification of genes involved use of the LC480 LightCycler Real Time PCR system (Roche Applied Science, Meylan, France) and the Go Taq QPCR master mix (Promega, Charbonnières les Bains, France). mRNA levels of MMP-3 and MMP-13, β-catenin (Ctnnb1) and Lef1 were normalized to that of hypoxanthine–guanine phosphoribosyltransferase (Hprt), used as the internal standard.
The primes used (sense and antisense) were as follows: Mmp-3, s-TGAAAATGAAGGGTCTTCCGG and as-GCAGAAGCTCCATACCAGCA; Mmp-13, s-GATGGCACTGCTGACATCAT and as-TGTAGCCTTTGGAACTGCTT; Ctnnb1, s-GCAGCAGCAGTTTGTGGA and as-TGTGGAGAGCTCCAGTACACC; Lef1, s-TCCTGAAATCCCCACCTTCT and as-TGGGATAAACAGGCTGACCT; and Hprt, s-AGGACCTCTCGAAGTGT and as-ATTCAAATCCCTGAAGTACTCAT.
MMP-3 and MMP-13 protein measurement
The amount of MMP-3 and MMP-13 released by chondrocytes in response to IL-1β was assessed in the culture supernatants. For total mouse MMP-3 secretion we used a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Lille, France). For total mouse MMP-13 secretion we performed western blot analysis as described previously [32] with rabbit polyclonal antibody for MMP-13 (H-230; Santa Cruz Biotechnology, Tebu-Bio). Densitometry analysis of immunoblots involved use of Multi Gauge software (Fujifilm, Paris, France).
Glycosaminoglycan and matrix metalloproteinase enzymatic activity assays
The amount of glycosaminoglycan and global MMP activity was measured in the culture supernatants of compressed cartilage explants. Released glycosaminoglycan was assessed by reaction with dimethylmethylene blue [33]. Shark chondroitin sulfate was used as a standard. MMP activity was assessed by Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 synthetic fluorogenic substrate (Bachem, Weil am Rhein, Germany) in continuous assays [34]. Results were normalized to milligram wet weight cartilage and concentration of proteins in the culture supernatant.
β-Catenin accumulation
Intracellular proteins were extracted from chondrocytes cultured with and without IL-1β. Assay of total mouse β-catenin involved western blot analysis with rabbit polyclonal antibody for β-catenin (Cell Signaling, Danvers, MA, USA). A reprobing with anti-β-actin antibodies (AC-15; Sigma Aldrich) served as a loading control. Densitometry analysis of immunoblots involved use of Multi Gauge software (Fujifilm). The ratio of β-catenin band intensity to the β-actin band intensity was calculated.
Statistical analysis
Data are expressed as the mean ± standard error of the mean and were analyzed by the use of Mann–Whitney nonparametric tests. Three to four independent experiments were performed. P values and number of experiments (n) are indicated in the figure legends.
Results
Load-induced catabolism is enhanced in FrzB−/− cartilage
Cartilage explants from FrzB−/− and wild-type mice were stimulated by dynamic compression for 6 hours. As previously published by our group, load induced cartilage degradation in the explants [35]. Cartilage degradation was revealed by a net increase in glycosaminoglycan release (2.8-fold, P = 0.014; Figure 1A) and MMP activity (4.5-fold, P = 0.014; Figure 1B). At baseline, cartilage degradation was not different between the FrzB−/− and wild-type samples (data not shown). Load-induced glycosaminoglycan release was similar in FrzB−/− and wild-type samples but load-induced increase in MMP activity was slightly enhanced in FrzB−/− cartilage explants compared with wild-type explants (1.6-fold, P = 0.17; Figure 1B). The lack of FrzB thus tends to enhance the catabolic response of chondrocytes in situ, in response to mechanical stress.
Load-induced glycosaminoglycan release and matrix metalloproteinase activity in mouse cartilage explants from FrzB−/−and wild-type mice. Explants were subjected to dynamic compression for 6 hours (0.5 Hz, 1 MPa). FrzB−/− cartilage explants (black bars, n = 4) or wild-type (WT) explants (gray bars, n = 4) were loaded. Results from the loaded cartilage explants were normalized to those from the corresponding nonloaded explants (Ctrl), so that the graphs represent the fold-induction in response to compression. (A) Amount of glycosaminoglycan (GAG) released from cartilage explants into culture supernatant. (B) Matrix metalloproteinase (MMP) enzymatic activity was measured in the culture supernatant of cartilage explants. Load-induced MMP activity tends to be enhanced in FrzB−/− explants compared with WT (P = 0.17). Bars represent the mean ± standard error of the mean. *P ≤ 0.05 versus Ctrl. FrzB, frizzled-related protein B; KO, knockout.
IL-1β-mediated MMP induction is enhanced in FrzB−/− chondrocytes
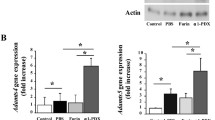
Primary cultures of articular chondrocytes from FrzB−/− and wild-type mice were stimulated with increasing doses of IL-1β for 24 hours (10, 100 and 1000 pg/ml). IL-1β dose-dependently induced Mmp-13 gene expression (up to 15-fold, P = 0.05; Figure 2A). At the higher dose, IL-1β-induced Mmp-13 gene expression was enhanced in FrzB−/− chondrocytes compared with wild-type chondrocytes (P = 0.05). Of note, in basal conditions Mmp-13 gene expression was similar in FrzB−/− and wild-type chondrocytes (0.012 ± 0.008 vs. 0.020 ± 0.008 arbitrary units, respectively). At protein level, MMP-13 release was measured by western blot analysis (Figure 2B). MMP-13 release was not modulated by the lower doses of IL-1β, but was significantly increased in response to 1,000 pg/ml (P = 0.014). This increase in MMP-13 release tended to be slightly enhanced in FrzB−/− chondrocytes compared with wild-type chondrocytes (1.5-fold, P = 0.17). Similarly, IL-1β dose-dependently induced Mmp-3 gene expression (up to 500-fold, P = 0.05; Figure 3A). At the higher dose, IL-1β-induced Mmp-3 gene expression tended to be enhanced in FrzB−/− chondrocytes compared with wild-type chondrocytes (P = 0.10). As for Mmp-13, Mmp-3 gene expression was similar in FrzB−/− and wild-type chondrocytes in basal conditions (0.0021 ± 0.0010 vs. 0.0017 ± 0.0004 arbitrary units, respectively). At protein level, MMP-3 release was assessed by enzyme-linked immunosorbent assay (Figure 3B). MMP-3 was not detected in untreated conditions, but only in response to the higher doses of IL-1β (P = 0.05). MMP-3 release in response to 100 pg/ml IL-1β was enhanced in FrzB−/− chondrocytes compared with wild-type (2.1-fold, P = 0.10). The absence of FrzB thus tends to enhance the catabolic response of cultured chondrocytes, in response to a proinflammatory stress. These results are parallel to the enhanced catabolic response observed in loaded FrzB−/− cartilage explants.
IL-1β-mediated increase in MMP-13 in cultured articular chondrocytes is enhanced in absence of FrzB. Primary chondrocytes were treated for 24 hours with interleukin (IL)-1β (10, 100 or 1,000 pg/ml). Results from the treated chondrocytes were normalized to those from nontreated samples (Ctrl), so that the graphs represent the fold-induction in response to IL-1β. (A) Real-time polymerase chain reaction analysis of Mmp-13 gene expression. IL-1β-induced Mmp-13 gene expression tends to be enhanced in FrzB−/− chondrocytes compared with wild-type (WT) chondrocytes, especially with 1,000 pg/ml (P = 0.05, n = 3). (B) Culture media were analyzed for MMP-13 by western blotting. Quantification of the MMP-13 blot is shown above the blot. IL-1β-induced MMP-13 protein release tends to be enhanced in FrzB−/− chondrocytes compared with WT chondrocytes, especially with 1,000 pg/ml (P = 0.17, n = 4). Bars represent the mean ± standard error of the mean. *P ≤ 0.05 versus Ctrl, #P ≤ 0.10 between FrzB−/− and WT. FrzB, frizzled-related protein B; KO, knockout; MMP, matrix metalloproteinase; ND, not detected.
IL-1β-mediated increase in MMP-3 in cultured articular chondrocytes is enhanced in absence of FrzB. Primary chondrocytes were treated for 24 hours with interleukin (IL)-1β (10, 100 or 1,000 pg/ml). (A) Real-time polymerase chain reaction analysis of Mmp-3 gene expression. Results from the treated chondrocytes were normalized to those from nontreated samples (Ctrl), so that the graphs represent the fold-induction in response to IL-1β. The IL-1β-induced Mmp-3 gene expression tends to be enhanced in FrzB−/− chondrocytes compared with wild-type (WT) chondrocytes, especially with 1,000 pg/ml (P = 0.10, n = 3). (B) Culture media were analyzed for total MMP-3 by enzyme-linked immunosorbent assay. MMP-3 protein release in response to 100 pg/ml IL-1β tends to be enhanced in FrzB−/− chondrocytes compared with WT chondrocytes (P = 0.10, n = 3). Bars represent the mean ± standard error of the mean. *P ≤ 0.05 versus Ctrl, #P ≤ 0.10 between FrzB−/− and WT. FrzB, frizzled-related protein B; KO, knockout; MMP, matrix metalloproteinase; ND, not detected.
IL-1β-induced and load-induced catabolism in FrzB−/− chondrocytes is associated with canonical Wnt/β-catenin signaling
We wondered whether the enhancement in the response of FrzB−/− chondrocytes to IL-1β and load was associated with a deregulation in Wnt/β-catenin signaling. In basal conditions, Ctnnb1 gene expression (coding β-catenin) was comparable between FrzB−/− cultured chondrocytes and wild-type chondrocytes (0.62 ± 0.22 vs. 0.95 ± 0.12 arbitrary units, respectively). Of note, similar results were observed for the Wnt/β-catenin target gene Lef1 (0.031 ± 0.009 for wild-type chondrocytes vs. 0.018 ± 0.013 for FrzB−/− chondrocytes). These data are in accordance with the previous transcriptome analysis of the bone–cartilage unit of FrzB−/− mice: at baseline in vivo, the Ctnnb1 mRNA level was not different between the FrzB−/− and wild-type strains [36].
Ctnnb1 gene expression was not affected by IL-1β in wild-type cultured chondrocytes (Figure 4A). In IL-1β-treated FrzB−/− chondrocytes, Ctnnb1 gene expression was induced 4.3-fold compared with untreated chondrocytes (P = 0.014). Ctnnb1 gene expression was thus markedly higher in IL-1β-treated FrzB−/− chondrocytes compared with wild-type chondrocytes (3.6-fold, P = 0.057). In parallel, Lef1 gene expression was induced 6.2-fold in IL-1β-treated FrzB−/− chondrocytes compared with untreated chondrocytes (P = 0.05) and the Lef1 mRNA level was clearly higher in IL-1β-treated FrzB−/− chondrocytes compared with wild-type chondrocytes (13-fold, P = 0.05; Figure 4A). Analysis of β-catenin accumulation at the protein level did not strictly correlate this transcriptional regulation (Figure 4B). IL-1β tended to decrease the β-catenin content of wild-type cultured chondrocytes (20% less, P = 0.057), whereas no modulation was observed in FrzB−/− chondrocytes. The β-catenin content was thus higher in IL-1β-treated FrzB−/− chondrocytes compared with wild-type chondrocytes (1.4-fold, P = 0.10). IL-1β only slightly modulates Wnt/β-catenin signaling in our model, but FrzB-dependent deregulation was observed in IL-1β-treated chondrocytes.
IL-1β-mediated and load-mediated regulation of Wnt/β-catenin signaling in chondrocytes from FrzB−/−and wild-type mice. Cultured articular chondrocytes from FrzB−/− mice or wild-type (WT) mice were treated for 24 hours with interleukin (IL)-1β (1 ng/ml). Results from the IL-1β-treated samples were normalized to the control ones (Ctrl), so that the graphs represent the fold-induction in response to IL-1β. (A) Ctnnb1 gene expression (coding β-catenin) and Lef1 gene expression (a Wnt/β-catenin target gene) were analyzed by real-time polymerase chain reaction (PCR; n = 4 and n = 3, respectively). Ctnnb1 gene expression was not modulated by IL-1β in WT chondrocytes and Lef1 gene expression was decreased (P = 0.05). In contrast, the treatment induced Ctnnb1 and Lef1 gene expression in FrzB−/− chondrocytes (P = 0.014 and P = 0.05, respectively). IL-1β-mediated regulation of β-catenin expression was thus different between FrzB−/− and WT (P = 0.057 for Ctnnb1, P = 0.05 for Lef1). (B) Intracellular extracts were analyzed for total β-catenin by western blotting. Quantification of β-catenin blot suggested that IL-1β-mediated β-catenin accumulation was different between FrzB−/− and WT (P = 0.10, n = 4). (C) FrzB−/− cartilage explants or WT explants were subjected to dynamic compression for 6 hours (0.5 Hz, 1 MPa). Ctnnb1 gene expression was analyzed by real-time PCR (n = 3). Results from the loaded cartilage explants were normalized to those from the corresponding nonloaded explants (Ctrl), so that the graphs represent the fold-induction in response to compression. Ctnnb1 gene expression was not affected by load in WT explants but was increased 2.7 fold in compressed FrzB−/− samples (P = 0.05). The load-induced increase in Ctnnb1 mRNA tends to be enhanced in FrzB−/− explants compared with WT explants (P = 0.20). Bars represent the mean ± standard error of the mean, *P ≤ 0.05 versus Ctrl, #P ≤ 0.10 between FrzB−/− and WT. FrzB, frizzled-related protein B; KO, knockout.
Concerning the catabolic response of FrzB−/− cartilage to load, analysis of Ctnnb1 gene expression showed very similar results (Figure 4C). The Ctnnb1 mRNA level was not affected by compression in wild-type explants. In compressed FrzB−/− samples, Ctnnb1 gene expression was induced 2.7-fold compared with uncompressed samples (P = 0.05). The modulation in Wnt/β-catenin signaling in response to load was thus slightly enhanced in FrzB−/− cartilage (P = 0.20; Figure 4C).
Overall, these results suggest that the enhancement in the response of FrzB−/− chondrocytes to IL-1β and load may be associated with an overstimulation of the canonical Wnt pathway.
Discussion
Enhanced responsiveness to mechanical stress in the absence of FrzB and involvement of the canonical Wnt/β-catenin pathway
We have demonstrated that load-induced MMP activity was enhanced in FrzB−/− cartilage explants. In addition, load stimulated Ctnnb1 gene expression in FrzB−/− explants. These results suggest that the load-induced catabolic response of chondrocytes may be in part mediated by the canonical Wnt pathway. The involvement of Wnt signaling in osteoblast responsiveness to mechanical stimulation was proven in various studies [37–42]. In particular, TOPGAL reporter mice showed that the canonical Wnt/β-catenin pathway was activated by mechanical stress in vivo and in cultured osteoblasts [43]. Furthermore, increasing basal β-catenin levels was shown to enhance the effects of mechanical stress [44]. Chondrocyte responses towards mechanical stimulation have been less studied. However, pressure-induced mechanical stress triggered β-catenin tyrosine phosphorylation in cultured chondrocytes [45], thereby probably releasing the molecule from adherens junctions and increasing its availability for intracellular signaling. Partial β-catenin nuclear translocation was also observed in response to tensile strain [27]. Furthermore, there was an additive effect of load and Wnt-3a on β-catenin translocation and on upregulation of Mmp-3 gene expression [27]. Of interest, a downregulation of FrzB was observed in human and mouse cartilage explants in response to mechanical injury, suggesting a de-repression of the canonical Wnt pathway [46]. Wnt/β-catenin signaling may thus be part of the signaling response leading to excessive catabolism and cartilage degradation in response to abnormal loading and FrzB may have a protective role on load-induced increase in MMP activity.
Enhanced responsiveness to IL-1β in the absence of FrzB and involvement of the canonical Wnt/β-catenin pathway
We demonstrated that IL-1β-mediated increases in Mmp-3 and Mmp-13 gene expression and protein release were enhanced in FrzB−/− chondrocytes. Similarly, IL-1β stimulated Ctnnb1 and Lef1 gene expression and β-catenin accumulation in FrzB−/− chondrocytes. Canonical Wnt pathway activation may thus enhance chondrocyte responsiveness to IL-1β. These results suggest a crosstalk between the canonical Wnt pathway, which includes β-catenin and FrzB, and the IL-1 pathway, which stimulates MMP expression in chondrocytes. In accordance with our results, activation of β-catenin signaling in cultured chondrocytes by Wnt-3a treatment potentiated IL-1β-mediated loss of proteoglycans [25]. Conversely, inhibition of β-catenin signaling by the use of Lef1 siRNA downregulated IL-1β-mediated increase in Mmp-13 gene expression [29]. Sost, a biologically active inhibitor of β-catenin signaling in chondrocytes, downregulated the IL-1α-mediated increase in Mmp-13 gene expression in cartilage explants and also reduced the loss of proteoglycans [47]. An alternative suggestion is that IL-1β treatment may induce production of canonical Wnt such as Wnt-7b, which in turn would activate β-catenin signaling [48]. In surprising contrast, in human chondrocytes β-catenin signaling was found to counteract IL-1β-mediated increase in MMP-3 and MMP-13 expressions [48]. In conclusion, the canonical Wnt pathway may be part of mechanisms leading to excessive catabolism in response to inflammatory stress and FrzB may have a protective role on IL-1-mediated increase in MMP expression in mouse chondrocytes.
Putative protective role of FrzB in osteoarthritis progression
In OA, cartilage breakdown is due to cleavage of matrix molecules in response to abnormal mechanical stress and to some degree of inflammation. Because our results suggest that FrzB may have a protective role on load-mediated and IL-1-mediated catabolic processes in mouse chondrocytes, we speculate that FrzB may have a protective role in OA. Our results are consistent with the increased cartilage loss observed in models of arthritis in FrzB−/− knockout mice [18]. Moreover, gene expression of Mmp-3 was upregulated in the cartilage of FrzB−/− mice with mBSA-induced arthritis compared with wild-type mice. However, FrzB−/− knockout mice did not develop spontaneous OA, and they did not show aberrant Mmp gene expressions in basal conditions, except for a twofold increase for MMP-3 [36]. FrzB may thus be involved in OA progression rather than OA onset. Although FrzB may interact directly with MMP-3 [18], its protective role is probably linked with a deregulation of the Wnt pathways. The recent identification of FrzB as a blocker of hypertrophic differentiation in articular cartilage [22] promotes the hypothesis of a protective role of FrzB in OA through the Wnt-mediated regulation of hypertrophic maturation.
Conclusions
Our results suggest that FrzB has a protective role on MMP induction in mouse chondrocytes. The results indicate a dual role of Wnt signaling in cartilage homeostasis, so that a controlled amount of Wnt signaling is necessary for maintenance of the articular cartilage, but an excess one is deleterious. Further investigations are needed to decipher the tight control of Wnt signaling in OA, in particular concerning the differentiation of OA chondrocytes towards hypertrophy.
Our results also add evidence to demonstrate that the canonical Wnt/β-catenin pathway is part of mechanisms leading to excessive catabolism and cartilage degradation in OA. However, the FrzB-dependent deregulation that we observed may involve both canonical and noncanonical Wnt signaling since FrzB is an inhibitor of both pathways. Little is known concerning the role of the noncanonical Wnt pathway in articular cartilage homeostasis and OA development. Recent data suggested that, in excess, Wnt-5a could stimulate degradation of the mature cartilage matrix via noncanonical pathways, while promoting normal differentiation in developing cartilage [49]. Additional investigations on Wnt regulation in OA should therefore equally explore canonical and noncanonical Wnt pathways.
Authors’ information
This work was supported by a grant from Fondation pour la Recherche Médicale. CB was supported by a Fondation pour la Recherche Médicale postdoctoral fellowship and by French state funds managed by the Agence Nationale de la Recherche within the Investissements d’Avenir programme under reference ANR-11-IDEX-0004-02.
Abbreviations
- FrzB:
-
frizzled-related protein B (secreted frizzled-related protein 3)
- IL:
-
interleukin
- LEF:
-
lymphoid enhancer binding factor
- LRP:
-
low-density lipoprotein receptor
- MMP:
-
matrix metalloproteinase
- OA:
-
osteoarthritis.
References
Nakamura Y, Nawata M, Wakitani S: Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am J Pathol. 2005, 167: 97-105.
Dell’accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C: Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 2008, 58: 1410-1421.
Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, Newham P, van den Berg WB: Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009, 60: 501-512.
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D: Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009, 24: 12-21.
Papathanasiou I, Malizos KN, Tsezou A: Bone morphogenetic protein-2-induced Wnt/beta-catenin signaling pathway activation through enhanced low-density-lipoprotein receptor-related protein 5 catabolic activity contributes to hypertrophy in osteoarthritic chondrocytes. Arthritis Res Ther. 2012, 14: R82-
Papathanasiou I, Malizos KN, Tsezou A: Low-density lipoprotein receptor-related protein 5 (LRP5) expression in human osteoarthritic chondrocytes. J Orthop Res. 2010, 28: 348-353.
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ: Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012, 492: 1-18.
Chun JS, Oh H, Yang S, Park M: Wnt signaling in cartilage development and degeneration. BMB Rep. 2008, 41: 485-494.
Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, Ferreira A, Ciesielski C, Carson DA, Corr M: Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004, 101: 9757-9762.
Min JL, Meulenbelt I, Riyazi N, Kloppenburg M, Houwing-Duistermaat JJ, Seymour AB, Pols HA, van Duijn CM, Slagboom PE: Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum. 2005, 52: 1077-1080.
Lane NE, Lian K, Nevitt MC, Zmuda JM, Lui L, Li J, Wang J, Fontecha M, Umblas N, Rosenbach M, de Leon P, Corr M: Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum. 2006, 54: 1246-1254.
Valdes AM, Loughlin J, Oene MV, Chapman K, Surdulescu GL, Doherty M, Spector TD: Sex and ethnic differences in the association of ASPN, CALM1, COL2A1, COMP, and FRZB with genetic susceptibility to osteoarthritis of the knee. Arthritis Rheum. 2007, 56: 137-146.
Lories RJ, Boonen S, Peeters J, de Vlam K, Luyten FP: Evidence for a differential association of the Arg200Trp single-nucleotide polymorphism in FRZB with hip osteoarthritis and osteoporosis. Rheumatology (Oxford). 2006, 45: 113-114.
Evangelou E, Chapman K, Meulenbelt I, Karassa FB, Loughlin J, Carr A, Doherty M, Doherty S, Gomez-Reino JJ, Gonzalez A, Halldorsson BV, Hauksson VB, Hofman A, Hart DJ, Ikegawa S, Ingvarsson T, Jiang Q, Jonsdottir I, Jonsson H, Kerkhof HJ, Kloppenburg M, Lane NE, Li J, Lories RJ, van Meurs JB, Näkki A, Nevitt MC, Rodriguez-Lopez J, Shi D, Slagboom PE: Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 2009, 60: 1710-1721.
Snelling S, Ferreira A, Loughlin J: Allelic expression analysis suggests that cis-acting polymorphism of FRZB expression does not contribute to osteoarthritis susceptibility. Osteoarthritis Cartilage. 2007, 15: 90-92.
Rodriguez-Lopez J, Pombo-Suarez M, Liz M, Gomez-Reino JJ, Gonzalez A: Further evidence of the role of frizzled-related protein gene polymorphisms in osteoarthritis. Ann Rheum Dis. 2007, 66: 1052-1055.
Kerkhof JM, Uitterlinden AG, Valdes AM, Hart DJ, Rivadeneira F, Jhamai M, Hofman A, Pols HA, Bierma-Zeinstra SM, Spector TD, van Meurs JB: Radiographic osteoarthritis at three joint sites and FRZB, LRP5, and LRP6 polymorphisms in two population-based cohorts. Osteoarthritis Cartilage. 2008, 16: 1141-1149.
Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP: Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007, 56: 4095-4103.
Baker-Lepain JC, Lynch JA, Parimi N, McCulloch CE, Nevitt MC, Corr M, Lane NE: Variant alleles of the Wnt antagonist FRZB are determinants of hip shape and modify the relationship between hip shape and osteoarthritis. Arthritis Rheum. 2012, 64: 1457-1465.
Bos SD, Slagboom PE, Meulenbelt I: New insights into osteoarthritis: early developmental features of an ageing-related disease. Curr Opin Rheumatol. 2008, 20: 553-559.
Wu Q, Zhu M, Rosier RN, Zuscik MJ, O’Keefe RJ, Chen D: Beta-catenin, cartilage, and osteoarthritis. Ann N Y Acad Sci. 2010, 1192: 344-350.
Leijten JC, van Blitterwijk CA, Karperien M, Emons J, van Gool S, Wit JM, Sticht C, Decker E, Rappold G, Uitterlinden A, Rappold G, Hofman A, Rivadeneira F, Scherjon S, Wit JM, van Meurs J, van Blitterswijk CA, Karperien M: GREM1, FRZB and DKK1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012, 2012: 34535-
Baker-LePain JC, Lane NE: Relationship between joint shape and the development of osteoarthritis. Curr Opin Rheumatol. 2010, 22: 538-543.
van der Kraan PM, van den Berg WB: Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration?. Osteoarthritis Cartilage. 2012, 20: 223-232.
Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M: Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008, 88: 264-274.
Oh H, Chun CH, Chun JS: Dkk-1 expression in chondrocytes inhibits experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2012, 64: 2568-2578.
Thomas RS, Clarke AR, Duance VC, Blain EJ: Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis. Arthritis Res Ther. 2011, 13: R203-
Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M: Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005, 280: 19185-19195.
Yun K, Im SH: Transcriptional regulation of MMP13 by Lef1 in chondrocytes. Biochem Biophys Res Commun. 2007, 364: 1009-1014.
Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Saffar JL, Jacques C: Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res Ther. 2006, 8: R135-
Gosset M, Berenbaum F, Thirion S, Jacques C: Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008, 3: 1253-1260.
Masuko-Hongo K, Berenbaum F, Humbert L, Salvat C, Goldring MB, Thirion S: Up-regulation of microsomal prostaglandin E synthase 1 in osteoarthritic human cartilage: critical roles of the ERK-1/2 and p38 signaling pathways. Arthritis Rheum. 2004, 50: 2829-2838.
Farndale RW, Sayers CA, Barrett AJ: A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982, 9: 247-248.
Houard X, Monnot C, Dive V, Corvol P, Pagano M: Vascular smooth muscle cells efficiently activate a new proteinase cascade involving plasminogen and fibronectin. J Cell Biochem. 2003, 88: 1188-1201.
Bougault C, Gosset M, Houard X, Salvat C, Godmann L, Pap T, Jacques C, Berenbaum F: Stress-induced cartilage degradation does not depend on the NLRP3 inflammasome in human osteoarthritis and mouse models. Arthritis Rheum. 2012, 64: 3972-3981.
Lodewyckx L, Cailotto F, Thysen S, Luyten FP, Lories RJ: Tight regulation of wingless-type signaling in the articular cartilage – subchondral bone biomechanical unit: transcriptomics in Frzb-knockout mice. Arthritis Res Ther. 2012, 14: R16-
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ: Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006, 281: 31720-31728.
Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE: Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007, 282: 20715-20727.
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L: Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009, 24: 1651-1661.
Jansen JH, Eijken M, Jahr H, Chiba H, Verhaar JA, van Leeuwen JP, Weinans H: Stretch-induced inhibition of Wnt/beta-catenin signaling in mineralizing osteoblasts. J Orthop Res. 2010, 28: 390-396.
Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A: Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun. 2010, 394: 755-759.
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T: Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012, 50: 209-217.
Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ: TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005, 20: 1103-1113.
Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J: Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008, 283: 29196-29205.
Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Salter DM: Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J Bone Miner Res. 2000, 15: 1501-1509.
Dell’Accio F, De Bari C, El Tawil NM, Barone F, Mitsiadis TA, O’Dowd J, Pitzalis C: Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther. 2006, 8: R139-
Chan BY, Fuller ES, Russell AK, Smith SM, Smith MM, Jackson MT, Cake MA, Read RA, Bateman JF, Sambrook PN, Little CB: Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011, 19: 874-885.
Ma B, van Blitterswijk CA, Karperien M: A Wnt/beta-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012, 64: 2589-2600.
Hosseini-Farahabadi S, Geetha-Loganathan P, Fu K, Nimmagadda S, Yang HJ, Richman JM: Dual functions for WNT5A during cartilage development and in disease. Matrix Biol. 2013, 32: 252-264.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CB, SP, XH, CJ and FB were responsible for conception and design. CB, SP, AP and LS were responsible for acquisition of data. CB, SP, XH, RJL, CJ and FB were responsible for analysis and interpretation of the data. CB, SP and AP were responsible for drafting of the article. XH, RJL, CJ and FB were responsible for critical revision of the article for important intellectual content. All authors were responsible for final approval of the article.
Carole Bougault, Sabrina Priam contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bougault, C., Priam, S., Houard, X. et al. Protective role of frizzled-related protein B on matrix metalloproteinase induction in mouse chondrocytes. Arthritis Res Ther 16, R137 (2014). https://doi.org/10.1186/ar4599
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar4599