Abstract
The aim of this study was to assess the endorsement of reporting guidelines in Korean traditional medicine (TM) journals by reviewing their instructions to authors. We examined the instructions to authors in all of the TM journals published in Korea to assess the appropriate use of reporting guidelines for research studies. The randomized controlled trials (RCTs) published after 2010 in journals that endorsed reporting guidelines were obtained. The reporting quality was assessed using the following guidelines: the 38-item Consolidated Standards of Reporting Trials (CONSORT) statement for non-pharmacological trials (NPT); the 17-item Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) statement, instead of the 5-item CONSORT for acupuncture trials; and the 22-item CONSORT extensions for herbal medicine trials. The overall item score was calculated and expressed as a proportion.One journal that endorsed reporting guidelines was identified. Twenty-nine RCTs published in this journal after 2010 met the selection criteria. General editorial policies such as those of the International Committee of Medical Journal Editors (ICMJE) were endorsed by 15 journals. In each of the CONSORT-NPT articles, 21.6 to 56.8% of the items were reported, with an average of 11.3 items (29.7%) being reported. In the 24 RCTs (24/29, 82.8%) appraised using the STRICTA items, an average of 10.6 items (62.5%) were addressed, with a range of 41.2 to 100%. For the herbal intervention reporting, 17 items (77.27%) were reported. In the RCT studies before and after the endorsement of CONSORT and STRICTA guidelines by each journal, all of the STRICTA items had significant improvement, whereas the CONSORT-NPT items improved without statistical significance.The endorsement of reporting guidelines is limited in the TM journals in Korea. Authors should adhere to the reporting guidelines, and editorial departments should refer authors to the various reporting guidelines to improve the quality of their articles.
Similar content being viewed by others
Introduction
In cases in which full reporting information is inaccessible, billions of dollars are wasted, bias is introduced, and research and the care of patients are detrimentally affected [1]. Well-designed and well-conducted randomized controlled trials (RCTs) represent the best available methodology for evaluating the effects of health care interventions. In general, they deliver reliable results that could inform future research or clinical practice. Poorly executed trials with inadequate methodologies are associated with bias and might produce exaggerated effects of an intervention [2]. A thorough assessment of a trial's design, management and analysis is vital to assess the quality and reliability of its published research. This type of assessment is only possible if the trial report presents the critical information required for such an appraisal. High-quality reporting in evidence-based research articles is crucial for the dissemination and implementation of research findings, and reporting guidelines are useful tools for increasing the comprehensiveness, accuracy, and transparency of research studies [3]. The lack of clear, transparent, and sufficiently detailed reporting of RCTs is a barrier to an adequate appraisal of the quality and applicability of published trials [4].
In the mid-1990s, in response to concerns regarding the quality of reporting in RCTs, an international group of researchers, statisticians, epidemiologists and biomedical editors developed the Consolidated Standards of Reporting Trials (CONSORT) statement [5]. This statement has been endorsed by the World Association of Medical Editors, the International Committee of Medical Journal Editors (ICMJE) and the Council of Science Editors [6]. The CONSORT statement is a comprehensive guideline for reporting RCTs, and it is associated with trial design and implementation improvements [7]. Since the development of the CONSORT statement, several extensions and elaborations have been included to include reporting requirements for different types of trials and interventions such as herbal interventions [8], non-pharmacological treatments [9], reporting of harm [10], inferiority and equivalence trials [11], pragmatic trials and Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) [12].
Almost 600 general and specialty journals endorse the CONSORT statement [13]. Although reporting guidelines play a central role in improving the quality of articles, there are considerable opportunities to improve the reporting of Korean clinical trials. There has been a considerable increase in the number of trials focused on traditional medicine (TM) and complementary and alternative medicine (CAM) in Korea [14]. The quality of Korean clinical medical research articles has consistently been a topic of discussion, and the guiding effect of TM journals should not be ignored; therefore, it is necessary to review the requirements outlined by Korean medical journals.
Considering these needs, several local studies assessing the endorsement of reporting guidelines by journals and authors are available [15–18]. Additionally, it is necessary to assess the TM journals in Korea that endorse reporting guidelines to determine whether the articles published in these journals provide satisfactory descriptions of the study design and intervention by adopting standards for all of the items in the reporting guides.
In this study, we aimed to investigate the extent to which Korean TM journals incorporated reporting guidelines into their instructions for authors. Any reference to the ICMJE was studied, and the quality of the reporting of RCTs in the TM journals that endorsed reporting guidelines was assessed.
Review
Methods
This study was reported in accordance with the guidelines from PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [19].
Selection of journals and RCTs
We included the TM journals from our previous article, which introduced all of the TM journals in a Korean medical database [20] and excluded those that did not have a website.
The instructions for the authors in each TM journal were downloaded, and the text referencing the CONSORT statement or other information relevant to the reporting guidelines for trials was examined. Additionally, we searched for any reference to the ICMJE’s Uniform Requirement for Manuscripts Submitted to Biomedical Journals. If the reporting guidelines were not referenced in the journals, we assumed that the journal had not adopted the reporting guidelines. We identified the RCTs in the journals that had adopted reporting guidelines by screening all of the issues. We retrieved the parallel group of RCTs included in the journals published after 2010 that had adopted reporting guidelines and assessed whether the use of reporting guidelines in the RCT reports was appropriate.
Data extraction
One author (JC) reviewed the websites of the TM journals. The instructions for authors, manuscript submission documents and relevant information for authors were extracted as data sources, including guidelines or instructions pertaining to the domains of editorial policy. The data included the journal name, website, ISSN, general policy references and reporting guidelines.
The screening of the title, abstract and full text of potentially relevant RCTs was completed by two authors (JC and JHJ). The full-text reports of the RCTs published in journals that referred to reporting guidelines were downloaded. Two authors (JC and JHJ) independently reviewed these RCT reports to assess the quality of reporting and to determine whether flow diagrams were included and subsequently validated by the third reviewer (BKK). Disagreements were resolved by consensus or by the third and fourth authors of this study (KHK and MSL).
To ensure correct interpretation, the experienced systematic reviewers dedicated an extensive amount of time to discussing all of the reporting statement guidelines and independently assessing and scoring the reporting quality. The data were collected using a standardized form. To maximize accuracy, the data extraction was performed at least twice for each article during several months. The approach and assumptions for determining the study quality were discussed extensively with the other authors. Disagreements were resolved by discussion.
Identification of reporting guidelines
The CONSORT group highly recommended that guideline users refer to the current version while writing or interpreting the reports of clinical trials [13]. In this study, the current version of the reporting guidelines was used to assess the quality of reporting in the TM journals that endorsed reporting guidelines. The extensions of the CONSORT statement were developed to provide additional guidance for RCTs with specific designs, data and interventions [13]. We attributed appropriate guideline tools for each TM intervention in the RCTs. The extension of the CONSORT statement for RCTs to non-pharmacological trials (NPT) [9] was based on the CONSORT guidelines, and for assessing an NPT the extension guidelines consider specific issues that might affect the treatment results (that is, surgery, technical interventions, rehabilitation, psychotherapy, behavioral interventions, implantable and non-implantable devices, and complementary medicine).
We selected the reporting guidelines as follows: the NPT extension of the CONSORT 2010 (38 items) [9], the CONSORT extension for herbal medicine RCTs (22 items) [8], and the 17-item STRICTA guideline that was designed to replace the 5 CONSORT items for acupuncture trials [12].
Data analysis
Each item was rated using a dichotomous scale (that is, ‘reported’ or ‘not reported’). The rating of ‘reported’ was recorded in cases in which relevant information was at least partially reported in the article. The rating of ‘not reported’ was recorded when relevant information was completely missing in the article.
The extracted variables included the publication and reporting characteristics as well as the items rated as ‘reported’. The data were analyzed using Microsoft 2010 and SPSS WIN 12.0 K (SPSS, Inc., Chicago, IL, USA). To assess the adherence to the CONSORT-NPT guideline items, we calculated the number and proportion of articles describing each item. The sum of the scores was converted to a percentage value for the reported items of each article (the proportion of each item = the number of reported items/the total items) and each section (the proportion of each section = the percentage of the sum of items of each section/the total items of each section). Additionally, before-and-after comparisons were performed to investigate whether the reporting quality of the RCTs was altered after the journal endorsed the reporting guidelines. We presented the percentages and percentage differences with binominal 95% confidence intervals after the journal adopted the CONSORT-NPT and STRICTA reporting guidelines.
Results
Selection of the studies for analysis
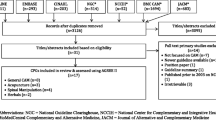
The selection process is presented in Figure 1. We identified 47 TM journals as potential candidates for our investigation of the adoption of reporting guidelines for clinical trials. Thirty-six journals were obtained from a Korean medical database. Of these journals, one journal recommends the use of reporting guidelines for clinical trials. To investigate the implementation of the reporting guidelines of the RCTs in this journal, the journal website was manually searched for all of the articles published from inception through December 2013.
After screening the abstracts and titles, we found 105 candidate articles. Subsequently, 76 articles were excluded because they were published before 2010 (55 trials). We additionally excluded the following: case studies (1), cross-over studies (7), reviews (11) and articles for other reasons (2). Lastly, 29 articles meeting our inclusion criteria were included, read completely and evaluated.
A total of 29 articles were eligible for the study. The types of interventions assessed in the 29 RCTs were as follows: acupuncture (24 trials), herbal medicine (1 trial), moxibustion (3 trials) and cupping (1 trial). The assessments were classified as non-pharmacological (96.6%) or pharmacological (3.4%).
General editorial policy
General editorial policies such as those of the ICMJE were endorsed by 15 journals (15/36, 41.6%); however, the journals did not specifically refer to reporting guidelines in the context of the ICMJE endorsement. Of the 15 journals, 2 journals specifically mentioned particular policies such as the Helsinki Declaration on Ethical Principles for Medical Research Involving Human Subjects, the Committee on Publication Ethics (COPE), and the Institutional Animal Ethical Committee (AEC) NIH Guide for the Care and Use of Laboratory Animals (Table 1).
Endorsement of reporting guidelines in TM journals
One (Journal of Korean Acupuncture and Moxibustion Medicine Society) of the 36 journals (1/36, 2.8%) referred to reporting guidelines in its instruction to authors (Table 1). The website of this journal provided the CONSORT guideline for reporting RCTs as well as the Systematic Reviews of Diagnosis Research (STARD) guideline, the STROBE guideline for observational studies, the Quality of Reporting of Meta-analyses (QUOROM) guideline, the Meta-analyses of Observational Studies (MOOSE) guideline and the Clinical Trial of Acupuncture Intervention (STRICTA) guideline from 2013.
Assessment of the reporting quality of the included RCTs
CONSORT 2010 with non-pharmacological trials
A total of 28 RCT reports were collected from the Journal of Korean Acupuncture & Moxibustion Medicine Society, which endorsed a reporting guideline. The majority of the 28 RCTs appraised using the 38-item guideline demonstrated a very low reporting quality because they addressed an average of 11.3 CONSORT items (29.6%). Among the 28 included articles, the reporting percentage in each of the articles was 21.6 to 56.8% (Table 2).
Of the 28 included articles, 3 (10.7%) mentioned ‘randomization’ in the title, and 2 (7.1%) described the experimental treatment, comparator, care providers, centers and blinding status. All of the articles (100%) described the scientific background and objective. More than 50% of the articles reported the participants (4a, 4b), the outcomes (6a) in the method section, the participant flow (13a), the implementation of the intervention (a new item in the CONSORT-NPT), the outcomes and estimations (17a) in the results section and the limitations (20) in the discussion section. The other items were assessed in less than 50% of the RCTs. Four items (7a, 7b, 12a and 12b) were not mentioned. The mean percentages for each article section were as follows: the title and abstract section, 8.93%; the introduction section, 100%; the method section, 29.31%; the results section, 40.26%; and the discussion section, 28.57%. The mean percentage for other information was 15.48% and for randomization, 9.82% (Table 3).
Of these RCT reports, 4 trials (14.3%) contained flow diagrams, and 3 (10.7%) provided information regarding the trial registration and protocol.
STRICTA 2010 for acupuncture interventions trials
The majority of the 24 RCTs (24/29, 82.8%) appraised using the 17 items scored more than 50% in reporting quality by addressing an average of 10.6 STRICTA items (62.5%). Using the STRICTA guidelines, the reporting percentage for each of the articles was 41.18 to 100%, and 2 articles (8.3%) reported all of the items (Table 2).
Among the 24 articles assessed using the 17 items on the STRICTA 2010 guideline, 23 (95.8%) reported the style of acupuncture and the name of the point used and provided a precise description of the control or comparator. A total of 87.5% described the needle type, 79.2% reported the reason for treatment and the needle retention time, 62.5% reported the depth of insertion, 58.3% reported a description of the participating acupuncturists, and 54.2% reported the number of treatment sessions.
The remainder of the items was reported in less than 50% of the RCTs. The mean reporting percentage was 62.5% for the acupuncture rational, 64.3% for the details regarding the reasons for acupuncture being needed, 66.7% for the treatment regimen, and 68.8% for the control or comparator interventions (Table 4).
The CONSORT extension for herbal intervention trials
One RCT was assessed using the recommendations for the reporting of herbal interventions. Of the 22 items, 17 items (77.27%) were reported. Of the 6 intervention items, the following 4 items (66.7%) were reported: the product name, dosage regimen and quantitative description, placebo/control group and practitioner.
Comparison after journal adoption of reporting guidelines
Table 5 compares the reporting of the guideline items in the RCTs from the journals that did or did not endorse the CONSORT and STRICTA guidelines. The percentages of the reported items in the RCTs (the percentage difference, 21.1; 95% CI, 6.6 to 35.5) were better after the adoption of the STRICTA reporting guidelines, with statistical significance (P = 0.0042). In the CONSORT-promoting journals, the completeness of the reporting increased slightly (percentage difference, 3.1%; 95% CI, -5.0 to 11.2) after endorsement of the guidelines by the journal, without a statistically significant difference (P = 0.4592) (Table 5).
Discussion
This study is the most comprehensive review of the reporting guidelines in Korean TM journals. We utilized a highly sensitive approach to the endorsement of reporting guidelines by Korean TM journals and conducted a complete assessment of the reporting quality of the trials published in journals that endorsed reporting guidelines. In this study, 36 core journals were selected after investigating the endorsement of reporting guidelines and the uniform requirements for manuscripts (URM) of these Korean TM journals. Our study has shown that most Korean TM journals provide little or no guidance regarding the information to report in describing research dependent on study design and interventions. One journal (the Journal of Acupuncture and Moxibustion Society) has referenced the reporting guidelines in its instructions for authors since 2013. Additionally, we found that the majority of the articles failed to follow the reporting guidelines. Fifteen journals mentioned the ICMJE or the URM.
An important aspect of RCT quality is related to the consideration of randomization; however, in our study, all of the items are in very low percentages (9.82%). We hypothesized that treatment in TM is a unique and complex intervention, and difficulties in blinding and allocation concealment are significantly influenced by the care providers’ expertise and the care center’s volume of treatment. A total of 4.3% of the studies referenced the trial registration number, protocol and funding source, which indicates journal editors have not focused on these items.
The Journal of Korean Acupuncture and Moxibustion Medicine Society is a core journal that is highly cited in Korea. In this journal, we found that 29 parallel RCTs of the 105 assessed RCTs did not report the number of care providers and centers in each group. For non-pharmacological treatments, the number of care providers and centers in each group and the number of patients treated by each care provider is essential so that the biological and statistical significance of the results could be assessed or the data reanalyzed. ‘Implementation of intervention’ is a new item added to the CONSORT-NPT 2010 statement. Because participants and care providers are frequently not blinded to treatment assignment in NPT, a risk for unequal administration of additional treatments and contamination might influence the estimates of the treatment effect [9]. In our study, 25 RCTs (89.3%) provided details regarding the manner in which the intervention was actually administered.
The CONSORT-NPT statement has not been translated into Korean; however, the STRICTA guidelines have been translated into Korean and were published in our endorsing journal in 2001 [50]. In addition, the translation could be found on the revised STRICTA website [51]. It is assumed that Korean journal editors and authors have simple access to the STRICTA items when reporting on acupuncture trials. Our findings showed that the STRICTA items (10.6/17, 62.5%) are more completely reported than the CONSORT-NPT items (11.28/37, 29.6%).
To adequately translate research into practice, research results should be reported by a method that is useful to practicing clinicians and policymakers. Based on evidence from systematic reviews, the implementation of reporting guidelines such as CONSORT for randomized controlled trials might improve the quality of research reporting. Other reporting guidelines similar to the CONSORT statement provide advice on reporting research methods and findings for other types of study designs [52].
Several studies [53–55] have assessed the effect of using the CONSORT statement to improve the reporting of RCTs. These studies suggested that journal endorsement of CONSORT might improve the completeness of reporting in the RCTs they publish. In our study, we found that RCTs published after reporting guidelines were adopted by journals were more likely to include the required reporting items shown in Table 5. Despite the relative improvements when CONSORT is endorsed by journals, the completeness of reporting remains suboptimal. In particular, Korean TM journals should guide their authors towards reporting guidelines, which have the potential to improve the quality of reporting and consequently the quality of research.
This study identifies several specific tasks required to improve the quality of clinical trials. First, all Korean TM Journals should be more vigilant regarding the information in their instructions to authors and explicit in their expectations of adherence to specific recommendations; additionally, they should cite the web address for the guidelines they follow to ensure that the latest versions and extensions are obtained. Second, in the initial submission stage, TM authors should adhere to the reporting guidance for the study design and intervention. Third, several reporting guidelines exist, and TM practitioners and researchers should consider the other interventions and develop the formal endorsement of guidelines for the TM intervention. Fourth, improved education and awareness among all of the stakeholders and hard-wired compliance through electronic journal submission systems could benefit the quality of clinical trials.
This study has the following limitations. First, because we found that only one Korean TM journal has adopted reporting guidelines, we retrieved only 29 RCTs for assessment, and the results could not fully represent all of the Korean TM journals. We could hypothesize that journals, RCTs or study designs not mentioned in this study have little potential for better reporting. This hypothesis is likely because many reporting guidelines have not been recommended by Korean TM journals. Second, our measures of study quality depend on the information reported in an article, and no attempt was made to judge the clinical merits or assumption models in the analyses. Third, no telephone calls were made and no Emails were sent to the editorial offices of the journals investigated; therefore, we do not know the opinions of the journal editors regarding the issues addressed in this study.
Our data suggest the need for TM journals to adopt reporting guidelines because better reporting is likely to influence the quality as well as the effect of future research. Korean researchers could provide robust evidence to establish health care standards for clinical practice. TM researchers in Korea should exert significant effort in improving the number and quality of primary studies by considering the study design and unique treatment intervention system in reference to reporting guidelines.
Conclusions
The endorsement of reporting guidelines is limited in TM journals in Korea, and many items in research studies were far from satisfactory. We hope that all of the TM journals will support reporting guidelines by registering on the websites of the organizations that have established reporting guideline statements. This article should generate further research regarding the mechanism for improving the quality of RCT reporting. Interested readers, reviewers, researchers, and editors in Korea could use the reporting guide statements and generate theories for improving research.
Abbreviations
- AEC:
-
Animal Ethical Committee
- CAM:
-
complementary and alternative medicine
- CONSORT-NPT:
-
Consolidated Standards of Reporting Trials - statement for non-pharmacological trials
- COPE:
-
Committee on Publication Ethics
- ICMJE:
-
International Committee of Medical Journal
- MOOSE:
-
Meta-analyses of Observational Studies
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUOROM:
-
Quality of Reporting of Meta-analyses
- RCTs:
-
randomized controlled trials
- STARD:
-
Systematic Reviews of Diagnosis Research
- STRICTA:
-
Standards for Reporting Interventions in Clinical Trials of Acupuncture
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- TM:
-
traditional medicine
- URM:
-
Uniform Requirements for Manuscripts.
References
Chan AW, Song F, Vickers A, Jefferson T, Dickersin K, Gøtzsche PC, Krumholz HM, Ghersi D, van der Worp HB: Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014, 383 (9913): 257-266. 10.1016/S0140-6736(13)62296-5.
Schulz KF, Grims DA: Lancet: Handbook of Essential Concepts in Clinical Research. 2004, London: Elsevier
Simera I, Altman DG, Moher D, Schulz KF, Hoey J: Guidelines for reporting health research: the EQUATOR network's survey of guideline authors. PLoS Med. 2008, 5 (6): e139-10.1371/journal.pmed.0050139.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG: CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010, 340: c869-10.1136/bmj.c869.
Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D: Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA. 1996, 276 (8): 637-639. 10.1001/jama.1996.03540080059030.
Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM: Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ. 2005, 330 (7499): 1057-1058. 10.1136/bmj.38413.576713.AE.
Schulz KF, Altman DG, Moher D, for the CONSORT Group: CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8: 18-10.1186/1741-7015-8-18.
Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, for the CONSORT Group: Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J Clin Epidemiol. 2006, 59 (11): 1134-1149. 10.1016/j.jclinepi.2005.12.020.
Boutron I, Moher D, Altman DG, Shultz KF, Ravaud P, for the CONSORT Group: Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008, 148 (14): 295-309.
Ioannidis JPA, Evans SJW, Gøtzsche PC, O’Neill RT, Altman DG, Schultz K, Moher D: Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004, 141 (10): 781-788. 10.7326/0003-4819-141-10-200411160-00009.
Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG, for the CONSORT Group. ftC: Reporting of noninferiority and equivalence randomized trials. Extension of the CONSORT 2010 statement. JAMA. 2012, 308 (24): 2594-2604. 10.1001/jama.2012.87802.
MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, Moher D, for the STRICTA Group: Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 2010, 7 (6): e1000261-10.1371/journal.pmed.1000261.
CONSORT Group: The CONSORT website. Available at: [http://www.consort-statement.org/]. Accessed 6 January 2013
Park HL, Lee HS, Shin BC, Liu JP, Shang Q, Yamashita H, Lim BM: Traditional medicine in China, Korea, and Japan: a brief introduction and comparison. Evid Based Complement Alternat Med. 2012, 2012
Hoffmann T, English T, Glasziou P: Reporting of interventions in randomised trials: an audit of journal Instructions to authors. Trials. 2014, 15 (1): 20-10.1186/1745-6215-15-20.
Tharyan P, George AT, Kirubakaran R, Barnabas JP: Reporting of methods was better in the Clinical Trials Registry-India than in Indian journal publications. J Clin Epidemiol. 2013, 66 (1): 10-22. 10.1016/j.jclinepi.2011.11.011.
Xiao L, Hu J, Zhang L, Shang HC: Endorsement of CONSORT by Chinese medical journals: a survey of ‘instruction to authors’. Chinese J Integr Med. 2014, 20 (7): 510-515. 10.1007/s11655-014-1865-8.
Scales CD, Norris RD, Keitz SA, Peterson BL, Preminger GM, Vieweg J, Dahm P: A critical assessment of the quality of reporting of randomized, controlled trials in the urology literature. J Urol. 2007, 177 (3): 1090-1094. 10.1016/j.juro.2006.10.027. discussion 1094-1095
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux P, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009, 151 (4): W-65-W-94.
Choi J, Lee JA, Yun KJ, Lim HJ, Lee MS: Online databases and journals of traditional medicine and complementary and alternative medicine in Korea. Eur J Integr Med. 2014, 6 (1): 64-73. 10.1016/j.eujim.2013.10.001.
Park JY, Kim MS, Jeon JC, Hwang HS, Jung KH, Lee TH, Roh JD, Lee EY: Comparative study of sosang(LU11)-eunbaek(SP1) bloodletting and sa-kwan(LI4 and LR3) acupuncture on acute dyspepsia. J Korean Acupunct Mox Med Sci. 2010, 27 (1): 129-135.
Lee HY, Lee JB, Cho YH, Song BY, Yook TH: The effects of Cervi pantotrichum cornu pharmacoacupuncture and Bovis calculus. Fel ursi pharmacoacupuncture on the heart rate variability. J Korean Acupunct & Mox Med Sci. 2010, 27 (1): 65-74.
Kim SH, Kim JS, Lee BH, Lim SC, Jung TY, Lee KM: Comparative clinical study of jung-an acupuncture and general acupuncture on Bell's palsy patients. J Korean Acupunct Mox Med Sci. 2010, 27 (1): 43-49.
Kwon HJ, Kim JK, Lee SH, Kim CH, Kim YS: Effect of shim-eui point on allergic rhinitis, rhinosinusitis, and other causes of nasal obstruction. J Korean Acupunct Mox Med Sci. 2010, 27 (3): 127-135.
Joung WJ, Wang KH, Kim KH, Bae JI, Kim SH, Cho HS: The effect of acupuncture at fengchi(GB20) and houzi(SI3) for acute headache due to whiplash injury. J Korean Acupunct Mox Med Sci. 2010, 27 (4): 127-135.
Yoon KS, Lee H, Kang JH, Choi JY: Comparison study on 30 cases of HIVD patients with restricted on SLRT by sa-am acupuncture banggwangjeonggy(膀胱正格) and general acupuncture. J Korean Acupunct Mox Med Sci. 2010, 27 (5): 79-87.
Kim SJ, Lee H, Jung HS, Kim ES, Woo JH, Han KW, Lee SJ, Lee JS, Yoo IS: A Clinical study on effect of electro-acupuncture treatment for lumbago patients caused by traffic accident. J Korean Acupunct Mox Med Sci. 2010, 27 (5): 117-123.
Chung JY, Kim JI, Lee SH, Kang SK: Effects of electroacupuncture on parameters related to obesity in adults with abdominal obesity: three arm randomised single blind pilot study. J Korean Acupunct Mox Med Sci. 2010, 27 (6): 43-57.
Choi YJ, Yoon KJ, Kim MS, Park JY, Jeon JC, Lee TH, Lee EY, Roh JD: Effects of scalp acupuncture with usual acupuncture on peripheral facial palsy in comparison with usual acupuncture only. J Korean Acupunct Mox Med Sci. 2010, 27 (6): 101-109.
Jang JY, Cho SY, Kim SJ, Kim YS, Nam SS: The effect of laser acupuncture at HT7(Sinmun) for mental stress on short-term analysis of heart rate variability. J Korean Acupunct Mox Med Sci. 2010, 27 (5): 51-58.
Kim MS, Park JY, Choi YJ, Yoon KJ, Jeon JC, Lee TH, Lee EY, Roh JD: Clinical effects of indirect moxibustion treatment with general acupuncture on HIVD patients in comparison with general acupuncture only. J Korean Acupunct Mox Med Sci. 2011, 28 (1): 65-75.
Park JY, Yun KJ, Choi YJ, Kim MS, Jeon JC, Lee TH, Lee EY, Roh JD: Comparative study of treatment effect between near acupuncture point needling and near acupuncture with remote acupuncture point needling on treatment of posterior neck pain. J Korean Acupunct Mox Med Sci. 2011, 28 (1): 85-92.
Park JS, Ahn MS, Lee JJ, Choi BS, Park MC, Yang HJ, Park GY, Kim MC, Jo EH: Study on the effect of acupuncture at jeonjung(CV17) on the heart rate variability in healthy adults. J Korean Acupunct Mox Med Sci. 2011, 28 (2): 13-25.
Lee JB, Im JG, Lee HG, Kim JU, Yook TH, Song BY: The comparison of effectiveness between acupuncture and its cotreatment with wan-gwa acupuncture on the treatment of low back pain. J Korean Acupunct Mox Med Sci. 2011, 28 (2): 43-47.
Jeong SY, Park ZW, Shin JM, Kim JY, Yae YI: The comparative study of effectiveness between acupuncture and its cotreatment with calculus Bovis. Fel Ursi. moschus pharmacoacupuncture on the treatment of acute low back pain. J Korean Acupunct Mox Med Sci. 2011, 28 (4): 105-110.
Shin HY, Lee SM, Kim JH, Kim SJ, Choi YJ, Jung TY, Kim JS, Lim SC, Lee YK, Lee BH, Lee KM: Comparative study of effects on intracutaneous bee venom pharmacopuncture and intramuscular bee venom pharmacopuncture in lumbar disc herniation. J Korean Acupunct Mox Med Sci. 2011, 28 (3): 1-11.
Lee CH, Ku JY, Park JA, Lee YH, Jang KJ, Song CH, Kim CH, Youn HM: Comparison of the efficacy between method of regulating ascending kidney water and descending heart fire and sweet bee venom pharmacopuncture on peripheral facial paralysis. J Korean Acupunct Mox Med Sci. 2011, 28 (4): 85-92.
Im JG, Lee JB, Lee HG, Lee TH, Kim JU: Effects of the acupuncture therapy in combination with soyeom pharmacopuncture therapy on acute whiplash injury by traffic accident. J Korean Acupunct Mox Med Sci. 2011, 28 (4): 9-18.
Kim MS, Park JY, Choi YJ, Yoon KJ, Lee CG, Lee EYDRJ: Comparative study of treatment effect between indirected moxibustion treatment with general acupuncture and general acupuncture only on treatment for neck pain caused by whiplash injury. J Korean Acupunct Mox Med Sci. 2011, 28 (6): 85-91.
Han SY, Lee JY, Park SH, Yang KY, Lee JH, Kim JS, Park JY, Kim CY, Lee HJ: A clinical study on effect of electro-acupuncture treatment for whiplash injury patients caused by traffic accident. J Korean Acupunct Mox Med Sci. 2011, 28 (6): 107-115.
Kim JE, Kang KW, Kim AR, Kim JH, Kim TH, Park HJ, Shin MS, Lee MH, Lee SH, Lee SH, Jung SY: Acupuncture for chronic fatigue syndrome and idiopathic chronic fatigue: a pilot randomised controlled trial. J Korean Acupunct Mox Med Sci. 2012, 29 (5): 109-118.
Kim SJ, Kim NS, Kim JY, Kim YS, Nam SS: Effect of acupuncture at yintang point(EX-HN3) on heart rate variability in healthy adults with mental stress. J Korean Acupunct Mox Med Sci. 2012, 29 (6): 47-56.
Kim JH, Jeong JY, Lee SW, Shin SY, Park JH, Kim CH, Jang KJ, Song CH, Yoon HM: Comparison of the efficacy between needle-embedding therapy and sweet bee venom pharmacopuncture therapy on peripheral facial paralysis. J Korean Acupunct Mox Med Sci. 2013, 30 (4): 35-44.
Kim KW, Yoo JH, Kim HH, Kim JH, Im SH, Chung IT, Kim JH, Lee JD, Choi DY: A controlled trial on the effect of complex oriental medical treatment with or without balanced acupuncture on treatment of herniated intervertebral disc of lumbar spine patients. J Korean Acupunct Mox Med Sci. 2013, 30 (4): 139-149.
Cho SY, Jang JY, Kim SJ, Nam SS, Kim YS: Effect of PC6 moxibustion for mental stress on short-term analysis of heart rate variability. J Korean Acupunct Mox Med Sci. 2010, 27 (2): 51-58.
Lee JJ, Kim SJ, Park OJ, Lee SM, Park MC, Jo EH: The effect of moxibustion at jeonjung(CV17) on the heart rate variability in healthy adults. J Korean Acupunct Mox Med Sci. 2012, 29 (4): 43-53.
Kim YR, Noh SH, Yang GY, Yook TH, Kim JU: The effects of different moxibustion stimulation at abdominal acupoints (CV12, CV6, CV4) on the skin temperature changes. J Korean Acupunct Mox Med Sci. 2013, 30 (1): 71-80.
Kim SJ, Park JS, Lee JJ, Park OJ, Kim SG, Jeong HH, Park MC, Kwon YM, Jo EH: The Effect of venesection with cupping therapy at (CV) on the heart rate variability in healthy adults. J Korean Acupunct Mox Med Sci. 2013, 30 (4): 15-24.
Song JY, Kim MJ, Sung WS, Kim PK, Goo BH, Kwak HY, Kim JH, Kim DH, Park YC, Seo BK, Baek HY, Choi DY, Lee JD, Park DS: Efficacy and safety of herb medication according to cold-heat tendency of knee osteoarthritis patients. J Korean Acupunct Mox Med Sci. 2012, 29 (5): 97-108.
Lee HS, Park JB, Seo JC, Park HJ, Lee HJ: Standards for reporting interventions in controlled trials of acupuncture: the STRICTA recommendations. J Korean Acupunct Mox Med Sci. 2001, 19 (6): 135-154.
Lee HS, Cha SJ, Park HJ, Seo JC, Park JB, Lee HJ: Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. Korean J Acupunct. 2010, 27 (3): 1-23.
EQUATOR Network: Enhancing the quality and transparency of health research. Available at [http://www.equator-network.org/]. Accessed 13 January 2014
Moher D, Jones A, Lepage L: Use of the CONSORT statement and quality or reports of randomized trials: a comparative before and after evaluation. JAMA. 2001, 285 (15): 1992-1995. 10.1001/jama.285.15.1992.
Devereux PJ, Mans BJ, Ghali WA, Quan H, Guyatt GH: The reporting of methodological factors in randomized controlled trials and the association with a journal policy to promote adherence to the Consolidated Standards of Reporting Trials (CONSORT) checklist. Control Clin Trials. 2002, 23 (4): 380-388. 10.1016/S0197-2456(02)00214-3.
Plint AC, Moher D, Morrison A, Schultz K, Altman DG, Hill C, Gaboury I: Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Syst Rev. 2006, 185 (5): 263-267.
Acknowledgements
The study was supported by the Korea Institute of Oriental Medicine (K14400).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MSL and JC conceived and designed the study. JC and JHJ extracted the information and assigned them into pre-defined categories. KHK and BKK analyzed data. JC wrote and revised the paper, with assistance from KHK and BKK. MSL coordinated the study. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Choi, J., Jun, J.H., Kang, B.K. et al. Endorsement for improving the quality of reports on randomized controlled trials of traditional medicine journals in Korea: a systematic review. Trials 15, 429 (2014). https://doi.org/10.1186/1745-6215-15-429
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1745-6215-15-429