Abstract
Background
The Mothers After Gestational Diabetes in Australia Diabetes Prevention Program (MAGDA-DPP) is a randomized controlled trial (RCT) that aims to assess the effectiveness of a structured diabetes prevention intervention for women who had gestational diabetes.
Methods/Design
The original protocol was published in Trial s (http://www.trialsjournal.com/content/14/1/339). This update reports on an additional exclusion criterion and change in first eligibility screening to provide greater clarity. The new exclusion criterion “surgical or medical intervention to treat obesity” has been added to the original protocol. The risks of developing diabetes will be affected by any medical or surgical intervention as its impact on obesity will alter the outcomes being assessed by MAGDA-DPP. The screening procedures have also been updated to reflect the current recruitment operation. The first eligibility screening is now taking place either during or after pregnancy, depending on recruitment strategy.
Trial registration
Australian New Zealand Clinical Trials Registry ANZCTRN 12610000338066.
Similar content being viewed by others
Update
Introduction
The Mothers After Gestational Diabetes in Australia Diabetes Prevention Program (MAGDA-DPP) is a randomized controlled trial (RCT) aiming to assess the effectiveness of a structured diabetes prevention intervention for women with previous gestational diabetes (GDM). The MAGDA-DPP trial offers an evidence-based structured lifestyle modification group intervention for such women. The objectives of MAGDA-DPP are that the intervention will result in favourable changes, relative to usual care, in clinical-, behavioural-, and patient-relevant outcomes. In addition, MAGDA-DPP aims to identify individual characteristics predictive of successful outcomes.
The MAGDA-DPP intervention is coordinated from Deakin University (Melbourne Campus). The project includes multiple study partners, consisting of two State Governments, three universities, and two non-government organizations. Ethical approvals have been obtained from multiple ethics authorities of the MAGDA project partners for the original study, with the lead ethics review authority being Deakin University Human Research Ethics Committee (reference number 2010–005).
The original protocol for the study has been presented by Shih et al. [1]. Following the publication of the protocol, amendments have been made to reflect the changes to initial screening and recruitment processes and provide greater protocol clarity. The two changes are outlined below. The amendments of the protocol have also been approved by Deakin University Human Research Ethics Committee in 2014. Informed consents have been obtained from all study participants.
Selection of participants
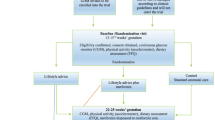
A new exclusion criterion has been added into the protocol “surgical or medical intervention to treat obesity”. Any medical or surgical intervention that impacts on the level of obesity, such as gastric banding, will alter the risks of developing diabetes and thus impact the outcomes being assessed by the MAGDA-DPP trial. Consequently, potential participants undergoing such an intervention will need to be excluded. The exclusion criteria are now: (i) pre-existing diabetes (Type 1 diabetes mellitus or T2DM); (ii) cancer (not in remission); (iii) severe mental illness; (iv) substance abuse (illicit drugs); (v) myocardial infarction in the last three months; (vi) difficulty with English; (vii) involvement in a post-natal lifestyle-based intervention or a trial which may impact primary clinical outcomes; (viii) pregnancy at post-natal baseline testing or at any point during the 12-months of study involvement; and (ix) surgical or medical intervention to treat obesity.
Additionally, the screening procedures have been updated to reflect current practice. As a result of slow recruitment initially, mail-outs were sent to women with recent GDM history in selected geographic areas. Following the mail-out, a large proportion of participants were recruited and their first eligibility screening was conducted over the phone at various post-partum time points. Therefore, the first eligibility screening took place either during or after pregnancy depending on recruitment strategy. This represents a change to the original protocol which stated “Once women diagnosed with GDM express interest in the study, the recruiter (MAGDA-DPP project manager or research assistant) will conduct the first eligibility screening generally prior to women delivering their babies”.
References
Shih STF, Davis-Lameloise N, Janus ED, Wildey C, Versace VL, Hagger V, Asproloupos D, O’Reilly S, Phillips PA, Ackland M, Skinner T, Oats J, Carter R, Best JD, Dunbar JA, MAGDA Research Group: Mothers After Gestational Diabetes in Australia Diabetes Prevention Program (MAGDA-DPP) post-natal intervention: study protocol for a randomized controlled trial. Trials. 2013, 14: 339-10.1186/1745-6215-14-339.
Acknowledgements
The study was funded by a Partnerships for Better Health Grant from the National Health and Medical Research Council (grant number: 533956) with financial and in-kind contributions from Victoria State Government, South Australia State Government, and Diabetes Australia Victoria. We would like to acknowledge Dr Rosalind Lau and Professor Prasuna Reddy for the initial development of the study protocol, as well as Kate Schlicht, Associate Professor John Reynolds, and Professor Catherine Bennett for their advice on aspects of psychology, statistics and epidemiology respectively. We would also like to acknowledge the contributions of the MAGDA Manual Training Committee and MAGDA RCT Working Group to the intervention handbooks and the study protocol revision. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of, nor should be attributed to, the National Health and Medical Research Council, Department of Health Victoria, South Australia Health, and Diabetes Australia Victoria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SS: Study design, manuscript writing, revision, and final approval of the version to be published; NDL: Study design, manuscript writing, revision, and final approval of the manuscript; EJ: Study design, obtaining funding for the study, revision, and final approval of the manuscript; CW: Amendment of the study protocol, manuscript writing, revision, and final approval of the manuscript; VV: Study design, revision, and final approval of the manuscript; VH: Study design, revision, and final approval of the manuscript; DA: Amendment of the study protocol, revision, and final approval of the manuscript; SO: Study design, revision, and final approval of the manuscript; PP: Study design, obtaining funding for the study, revision, and final approval of the manuscript; MA: Study design, obtaining funding for the study, revision, and final approval of the manuscript; TS: Study design, revision and final approval of the manuscript; JO: Study design, obtaining funding for the study, revision, and final approval of the manuscript; RC: Study design, obtaining funding for the study, revision, and final approval of the manuscript; JB: Study design, obtaining funding for the study, revision, and final approval of the manuscript; and JD: Study design, obtaining funding for the study, general supervisor of the study, revision, and final approval of the manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shih, S.T., Davis-Lameloise, N., Janus, E.D. et al. Mothers After Gestational Diabetes in Australia Diabetes Prevention Program (MAGDA-DPP) post-natal intervention: an update to the study protocol for a randomized controlled trial. Trials 15, 259 (2014). https://doi.org/10.1186/1745-6215-15-259
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1745-6215-15-259