Abstract
Reviewers
This article was reviewed by Neil S. Greenspan and Rachel Gerstein.
Nucleotides and nucleosides act as potent extracellular messengers via the activation of the family of cell-surface receptors termed purinergic receptors. These receptors are categorized into P1 and P2 receptors (P2Rs). P2Rs are further classified into two distinct families, P2X receptors (P2XRs) and P2Y receptors (P2YRs). These receptors display broad tissue distribution throughout the body and are involved in several biological events. Immune cells express various P2Rs, and purinergic signaling mechanisms have been shown to play key roles in the regulation of many aspects of immune responses. Researchers have elucidated the involvement of these receptors in the host response to infections. The evidences indicate a dual function of these receptors, depending on the microorganism and the cellular model involved. Three recent reports have examined the relationship between the level of extracellular ATP, the mechanisms underlying purinergic receptors participating in the infection mechanism of HIV-1 in the cell. Although preliminary, these results indicate that purinergic receptors are putative pharmacological targets that should be further explored in future studies.
Similar content being viewed by others
Introduction
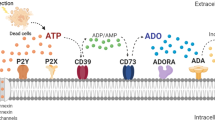
Nucleotides and nucleosides are fundamental molecules in cell metabolism that perform a wide range of acknowledged functions that include acting as energy sources and being the structural building blocks of nucleic acids [1, 2]. Furthermore, these compounds act as potent extracellular signaling molecules and neurotransmitters via the activation of a family of cell-surface receptors termed purinergic receptors [3]. Based on biochemical, pharmacological and molecular biological studies, purinergic receptors are categorized into P1 receptors (for adenosine) and P2 receptors (P2Rs, for ATP/ADP and some pyrimidines) [4–8].
To date, four P1 receptors have been identified: A1, A2A, A2B and A3. All of these molecules are typical G protein-coupled receptors, although they differ with respect to the G protein to which they are coupled [8, 9]. The P2Rs are separated into two distinct families: the ionotropic P2X receptors (P2XRs) (activated by ATP) and the metabotropic P2Y receptors (P2YRs) (that bind to ATP, UTP or their metabolites) [10–12]. Mammals express seven subtypes of P2XR monomers (P2X1-7R) and eight subtypes of P2YR monomers (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R) [13, 14].
The P2XRs function as ATP-gated ion channels. Following the binding of an agonist to their extracellular portions, they undergo conformational changes that result in the opening of an ion channel, facilitating the influx/efflux of cations [15–17]. Following prolonged activation by ATP, some P2XRs, such as P2X2R, P2X4R and P2X7R, are capable of undergoing an additional conformational change, which increases the permeability of large molecules [18–20]. In response to prolonged exposure to ATP, other P2XRs, such as P2X1R and P2X3R, undergo a fast desensitization, resulting in channel closure [17, 21–23]. Functional P2XRs are assembled into either homomeric or heteromeric trimers, each subunit of which contains two transmembrane domains; a large extracellular loop, which includes 10 conserved cysteines and glycosylation sites; and intracellular N and C terminal domains, which contain consensus phosphorylation sites for protein kinases [5, 6, 24–26]. The detailed signaling process that is triggered by the activation of most P2XRs has yet to be completely elucidated. For instance, one possible intermediate in the activation of MAPKs, PKC and calmodulin may be the cytoplasmic calcium [25, 27].
The P2YR subtypes are typical G protein-coupled receptors (GPCRs), which typically consist of seven transmembrane domains connected by three extracellular and three intracellular loops. The N-terminus is extracellular, and the C-terminus is intracellular [28–30]. Similar to other GPCRs, stimulation of P2YRs induces the activation of a heterotrimeric G protein and its dissociation into α and βγ subunits, activating a range of effector proteins, such as phospholipase C and adenylyl cyclase [25]. The P2YR family displays high diversity with respect to amino-acid composition and structural diversity with respect to the intracellular loops and the C-terminus, influencing the degree of coupling with functionally distinct types of G proteins [31, 32].
P2Rs are broadly distributed throughout nervous, endocrine, cardiovascular, renal, gastrointestinal and immune tissues [33] and participate in several biological events, such as platelet aggregation, exocrine and endocrine secretion, endothelial-mediated vasodilatation, nociceptive mechanosensory transduction, neuromodulation and neuroprotection, cell proliferation, differentiation, migration and death during embryological development, wound healing, epithelial cell turnover and immune responses [14, 16].
Review
Importance of purinergic signaling in immune responses
Most immune cells express both P1 and P2 receptor subtypes [34–39]. A large body of evidence has demonstrated that extracellular nucleotides and purinergic receptors are key regulators of many immune phenomena, such as inflammatory pain, cytokine secretion, chemotaxis, activation of various types of immune system cells, surface-antigen shedding and pathogen killing.
ATP, adenosine and other nucleotides are also involved in regulating the migratory responses of neutrophils, macrophages and other innate immune cells [40, 41]. Metabolic stress, ischemia, hypoxia, inflammation and trauma lead to the accumulation of adenosine in the extracellular space, reporting tissue injury to the surrounding tissue in an autocrine and paracrine manner. Another possibility is that adenosine generates tissue-protective responses [42–44]. In its protective function, adenosine downregulates the expression of adhesion molecules, the production of oxygen radicals, degranulation and the release of cytokines, consequently reducing cellular cytotoxic activities [45–52]. In particular, ATP and UTP that have been released by apoptotic cells act as “find-me signals” that induce phagocyte migration toward these cells [53]. In neutrophils, stimulation with the chemotactic peptide N-formyl-Met-Leu-Phe causes a rapid release of ATP in a polarized manner. This released ATP activates P2Y2R to modulate cellular orientation. The adenosine formed via ATP degradation and subsequent stimulation of the A3 receptor enables autocrine signal amplification, which facilitates chemotaxis by regulating the speed of migration [54]. Similar results have been observed in macrophages, in which stimulation with the chemoattractant C5a induced migration via the release of ATP and the activities of the autocrine purinergic signaling system [55]. In addition, some studies have suggested that ATP acts as a signal that induces the release of chemotactic factors [56]. In contrast, other studies have found evidence that nucleotides themselves function as chemotactic signals [53, 57, 58]. Extracellular nucleotides are also involved in modulating lymphocyte responses. B cells express various P2R subtypes [38, 59] and are able to release ATP under steady-state conditions [60]. Incubating human B cells with increased ATP concentration triggers a dose-dependent increase in the level of inositol 1,4,5-trisphosphate as well as an increase in the level of cytosolic free Ca2+. In addition, the c-fos and c-myc mRNA levels increase, indicating that P2-receptor stimulation was associated with this activity [61]. A transfection study using two P2X7R non-expressing human lymphoid cell lines (K562 and LG14 cells) showed that the heterologous expression of this receptor enhanced cell proliferation in the absence of growth factors and was dependent on the level of released ATP [62].
Similarly, several stages of the life of a T lymphocyte may be affected by purinergic signaling, including differentiation, activation and proliferation. Thymocytes are susceptible to apoptosis in the presence of extracellular ATP via the activation of purinergic receptors in vitro and in vivo [63–69]. The sensitivity of this cell type to extracellular ATP is closely associated with its degree of maturation, highlighting the importance of the purinergic system in the regulation of cellular differentiation in the thymus [70]. Recently, signaling via purinergic receptors has been associated with fate determination during T-cell development. P2X7R activation contributes to the strength of the γδTCR signal in immature thymocytes. The genetic ablation or pharmacological inhibition of this receptor directs cells toward the αβ fate [71]. Peripheral T lymphocytes release ATP into the extracellular milieu under several conditions, such as TCR stimulation, mechanical stimulation or osmotic stress [72–76], via either the pannexin 1 hemichannel [77, 78] or vesicular exocytosis [79]. In these cells, the binding of extracellular ATP to P2Rs modulates several steps required for complete T-cell activation, such as the influx of extracellular calcium, the activation of p38 MAPK and the secretion of IL-2 [72, 75, 80–82]. In addition to conventional αβ T lymphocytes, the activation state of γδ T cells is also regulated by ATP release and autocrine signaling via a purinergic receptor, namely P2X4R [83]. Similarly, activation of P2X7R by ATP inhibits the suppressive function and disrupts the gene transcription profile of Tregs and promotes the differentiation of these cells into proinflammatory TH17 effector cells [84].
Furthermore, purinergic signaling, particularly that involving P2X7R, also acts as a potent mediator of the release of proinflammatory cytokines. One of the best studied examples of this correlation is the participation of P2X7R in the processing and release of IL-1β and IL18 [85, 86]. Based on experiments performed in vitro and in vivo using P2X7-/- mice, ATP was identified as a strong IL-1β-releasing agent that acts via this receptor. LPS, the strongest inducer of IL-1 secretion, is an incomplete stimulus in the absence of ATP, leading to the accumulation of pro-Il-1β in cytoplasmic vesicles [85, 87].
These findings reinforce the importance of purinergic signaling mechanisms as key regulators of many aspects of immune responses. Under normal conditions, extracellular ATP (the natural agonist of most P2Rs) is present at nanomolar concentrations, which are maintained by extracellular nucleotide-hydrolyzing enzymes, such as ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) [88–90]. However, under certain conditions, such as inflammatory, ischemic and hypoxic states, several different cell types release ATP from intracellular storage compartments into the extracellular media, elevating its external concentration to the millimolar range and inducing predominantly proinflammatory responses [90, 91]. Several mechanisms for ATP release have been described, including cell death, vesicular transport, and the activities of stretch-activated channels, volume-regulated channels, maxi-anion channels, pannexin and connexin hemichannels and P2X7R [9, 13, 92]. In a “stressful environment” containing damaged host cells and the leakage of intracellular contents, purinergic receptors allow immune cells to recognize the ATP that is released into the extracellular milieu. Therefore, purinergic signaling participates in an important system that recognizes “danger” signals, alerting the immune system to a threat [93, 94]. Accordingly, the damage caused by pathogens at the infection site induces the release of additional ATP [95].
In this regard, it appears that a natural consequence of stress conditions is the alteration of purinergic signaling, which may be related to pathological processes associated with infectious conditions.
Involvement of purinergic receptors in HIV infection
Accordingly, some studies have investigated the involvement of purinergic receptors, particularly P2X7R, in infectious processes [96], as shown in Table 1.
Several studies have reported the involvement of purinergic receptors in viral infections, either as facilitators or as host defense factors. Purinergic receptor antagonists, such as suramin, pyridoxal-phosphate-6-azophenyl-2’ ,4’-disulfonate (PPADS) and brilliant blue G (BBG), the latter of which is a selective blocker of P2X7R, have been used to block the infection of hepatocytes by the hepatitis B virus [120–122]. Suramin has been demonstrated to exert antiviral effects on other animal viruses [123, 124], although no further studies have associated the antagonistic action of this compound with purinergic receptors. Moreover, human endothelial cells infected in vitro with cytomegalovirus displayed significantly increased expression of P2Y1R, P2Y2R and P2X7R compared to uninfected cells, although only slight effects were found following infection with the herpes virus, likely indicating a virus-specific effect [125].
Despite all of the efforts to control and prevent the spread of human immunodeficiency virus (HIV) type 1 (HIV-1), HIV-1 infection and the resulting acquired immunodeficiency syndrome (AIDS) remain public health problems worldwide. HIV is an enveloped virus classified into a subgroup of retroviruses termed lentiviridae because these viruses exhibit extended clinical latency and persistent viral replication throughout the illness [126–130]. The continuous replication of HIV-1 and chronic immune activation mediate the drastic depletion of CD4+ T cells, a hallmark of infection with HIV-1 and other associated immune disorders [131–134]. Throughout the course of infection, immune responses only partially control the level of these viruses in the blood [135].
The emergence of highly active antiretroviral therapy (HAART) has enabled significant improvements in the management of HIV-infected patients: the reduction of plasma HIV-1 levels below the level of detection of commercially available tests and limited immune reconstitution [136–139]. The use of HAART has resulted in a significant reduction in AIDS-related morbidity and mortality [140]. However, although HAART efficiently delays the onset of AIDS, its clinical utility is limited by several barriers, such as viral resistance, non-adherence to therapy and drug toxicity. Therefore, the search for new targets of HIV-1 and/or host cellular proteins is essential for the success of HIV-1 treatment [141–143].
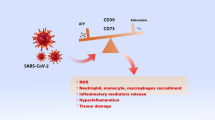
Three recent reports have directly assessed the possible involvement of purinergic receptors in the immunopathogenesis of HIV-1 infection, raising the possibility of using these host proteins as targets for HIV-1 treatment. Séror et al. found that infecting human cells with HIV-1 leads to the release of ATP via pannexin-1 hemichannels and that this event is essential for the initial infection. The ATP-degrading enzyme apyrase prevents HIV-1 infection. Similarly, pharmacological inhibition of pannexin-1 hemichannels or depletion of this protein using small interfering RNAs protected the targeted cells from HIV-1-mediated cell death and prevented HIV-1 infection. General inhibitors of purinergic receptors blocked the replication of X4-tropic and R5-tropic HIV in activated T lymphoblasts and that of R5-tropic HIV in macrophages and dendritic cells. The selective depletion of mRNAs encoding diverse purinergic receptors using interfering RNAs facilitated the identification of P2Y2R as a receptor related to HIV infection. Immunohistochemistry has revealed elevated levels of P2Y2R in lymphoid tissue, the frontal cortex and circulating leukocytes in untreated carrier patients compared with uninfected patients. Immunofluorescence microscopy has shown that P2Y2R is polarized at the virological synapse. Pharmacological inhibition or genetic ablation of P2Y2R has reproduced the effects of general purinergic receptor blockers. Ultimately, proline-rich tyrosine kinase 2 (Pyk2), a downstream effector of P2Y2R, was found to be a critical mediator of HIV-1 infection [144].
The second study demonstrated that inhibiting the purinergic receptors of macrophages resulted in a significant reduction in the rate of HIV replication. Macrophages are indispensable in HIV pathogenesis because they are susceptible to productive infection, often in the absence of cytopathic or deleterious effects, thus serving as long-lived virus reservoirs [145, 146]. Therefore, improving the understanding of the mechanisms by which HIV infects and replicates within macrophages is crucial for designing appropriate strategies to reduce the latency and spread of HIV-1 [147]. Using oxidized ATP, a P2XR antagonist, Hazleton et al. detected significant inhibition of HIV replication in macrophages in a dose-dependent and viral strain-independent manner, indicating that purinergic receptors may be required for HIV replication. To further discriminate which P2Rs may be involved, macrophages were treated with specific pharmacological P2R antagonists. This approach revealed the requirement of at least three purinergic receptors, P2X1R, P2X7R and P2Y1R. Moreover, using a β-lactamase HIV entry assay, P2X1R was demonstrated to participate in the viral entry of macrophages. Finally, treating primary cultures of human macrophages with HIV gp120 resulted in significantly increased release of ATP, indicating the possible autocrine regulation of critical events in the viral life cycle [148].
In the third study, P2XR antagonists inhibited HIV-1 from infecting CD4+ T lymphocytes via both cell-free and cell-to-cell contact in a dose-dependent manner. Additionally, exploration of a library of purinergic antagonists demonstrated that P2XR antagonists are the most potent inhibitors of HIV-1 fusion, providing evidence of a new therapeutic target to prevent HIV-1 infection of CD4+ T lymphocytes [149].
Purinergic receptors have also been associated with the neurotoxicity caused by HIV in infected individuals. In the central nervous system, microglia are activated by HIV-1, and in response, these cells release neuroinflammatory and excitotoxic products that are associated with the pathogenesis of neuroAIDS in many infected individuals [150]. Because P2XRs are key regulators of microglial functions, Sorrel and Hauser investigated whether these receptors are involved in the neurotoxic effects of HIV and morphine in infected individuals, as it has been reported that opioid-dependent microglial activation is mediated by P2X4R signaling. Pretreatment of microglia with TNP-ATP, a nonselective stimulator of P2XRs, inhibited Tat- and/or morphine-related neuronal death in a dose-dependent manner and prevented the increase in cytosolic free Ca2+. They also detected a rise in the level of ATP in the medium of neuron-glia co-cultures after 30 minutes of incubation in Tat and morphine, either individually or in combination. Finally, using P2XR antagonists and agonists, P2X4R was identified as the receptor responsible for neurotoxicity in HIV-infected individuals [151]. Similarly, large amounts of ATP, ADP and AMP and small amounts of adenosine and glutamate were detected in the supernatant of HIV-infected macrophage cultures. Applying diluted aliquots of these supernatants to neuronal cultures increased the amount of extracellular glutamate and decreased the neuronal spine density via mechanisms that are dependent on purinergic and glutamatergic receptor activation [150].
Previous investigations have also explored the role of adenosine receptors in the pathogenesis of AIDS. Pingle et al. demonstrated a protective effect of stimulating the A1A receptor (A1AR) against HIV-1 Tat-induced toxicity in primary cultures of rat cerebellar granule neurons and in rat pheochromocytoma (PC12) cells. Activation of A1AR ameliorated the Tat-mediated changes in PC12 cells, such as the increase in the intracellular Ca2+ content, the release of NO and the expression of inducible nitric oxide synthase (iNOS). Furthermore, pretreatment with an A1AR agonist reduced the level of activation of NF-κB by Tat and the number of apoptotic cells [152]. Moreover, A2AR stimulation inhibited the Tat-induced production of TNF-α, a cytokine that plays a pathological role in HIV-associated dementia, by macrophages [153]. Together, these studies suggest that modulating the activity of the adenosine receptor may be helpful in preventing HIV-1-associated abnormalities [154]. Furthermore, another report showed that stimulating the A2A receptor using a monoclonal antibody reduced the level of expression of the chemokine receptors CXCR4 and CCR5 in CEM T-cells, indicating a putative mechanism that could be exploited to block the entry of HIV-1 [155].
Conclusion
Purinergic receptors are considered to be powerful modulators of several physiological and pathological events, making them attractive molecular targets for pharmacological research. Although great advances in the management of HIV-infected patients have been achieved due to the emergence of HAART, barriers such as drug resistance, drug toxicity and the cost of treatment necessitate the development of new antivirals [156]. Accordingly, recent reports of the role of purinergic receptors in the immunopathogenesis of HIV-1 infection indicate that they are putative pharmacological targets that should be further explored. In addition, ATP could strengthen the function of the innate and adaptive immune systems because it modulates immunological events that are crucial for the anti-HIV response. For instance, prostaglandin E2 (PGE2) inhibited HIV-1 replication in macrophages via a protein kinase A-dependent mechanism [157]. Interestingly, P2X7R stimulation by ATP is required for the release of PGE2 and other autacoids [158]. Additionally, Leal and colleagues found a significant increase in the expression level of CD39 (NTPDase-1) in lymphocytes from HIV-patients, which correlated to a significant increase in the ATP- and ADP-hydrolytic activities [159]. These findings indicate that extracellular nucleotides might be closely associated with the immune response to HIV infection.
Therefore, further studies must be conducted to improve the understanding of the extent and impact of purinergic signaling activities during all stages of HIV-1 infection.
First round
Reviewer’s report
Reviewer 1: Neil S. Greenspan, Case Western Reserve University, United States of America
Reviewers’ comments
Pacheco et al. review several aspects of purinergic signaling including the receptors and ligands and their roles in immune responses, bacterial infections, protozoal infections, and HIV infection. A quick survey on PubMed of recent reviews on purinergic signaling reveals none focused precisely on the roles of purinergic ligands and receptors in HIV infection. Thus, the role of purinergic signaling in HIV infection is a topic deserving of review.
The sections on the effects of purinergic signaling in the context of either bacterial or protozoal infections are too brief to be of much use and distract from the main focus on the connections between purinergic signaling and HIV pathogenesis. Similarly, at the beginning of the section pertaining to HIV infection, mentions of effects of purinergic receptor antagonists on infection by “hepatitis virus” (which one, A?, B?, C?, D?) or the effects of cytomegalovirus on expression of purinergic receptors seem gratuitous and can be deleted.
Answer 1: We did the alterations recommended and focused the text in the HIV-1.
The focus needs to be more specifically on how purinergic ligands and receptors participate in HIV pathogenesis. Greater integration of the material addressing effects of purinergic molecules on immune responses in general and on HIV infection in particular would strengthen the article. The authors would also add value to their review by identifying specific experimental questions deserving further exploration.
Answer 2: We did the alterations recommended.
Quality of written English: Not suitable for publication unless extensively edited.
Answer 3: We sent the paper to American Journal Experts. The certificate is attached.
Reviewer’s report
Reviewer 2: Rachel Gerstein, University of Massachusetts Medical School, United States of America
Report form:
Overall, this review needs considerable revision to be more effective and readable.
Some specific comments that might guide the revision –
Abstract: An abstract should stand alone, and not require specialized knowledge to be understood by the reading audience. And most important, it must give a good idea of what the article is about. To state that there is a “relationship between […] purinergic signaling […] and immunopathogenesis of HIV-1” is very vague.
Answer 1: We did the alterations recommended.
Intro and body of review:
-
1.
Is purinergic signaling important in immune **responses** ? is ATP a DAMP for mature functional immune cells ? I was not convinced.
Answer 2: We did the alterations recommended.
-
3.
The section on thymocytes does not seem relevant to a discussion of response to a virus.
Answer 3: In the process of HIV-1 infection, one of the consequences is the reduction of the timopoiesis. The maturation and differentiation of thymocytes are modulated by IL-7 / IL-7 receptor (IL-7R) signaling pathway. During infection by HIV-1 occurs reduction in the level of IL-7 (Young and Angel, 2011).
Young CD 1 , Angel JB. HIV infection of thymocytes inhibits IL-7 activity without altering CD127 expression. Retrovirology. 2011 Sep 16;8:72. doi: 10.1186/1742-4690-8-72.
-
4.
The mention of TH17 profile, the context (ie “this condition”) is unclear.
Answer 4: We changed this in the text.
-
5.
If the authors want to highlight work most related to HIV-1, the inclusion of the sections on bacterial and protozoan infections are not needed.
Answer 5: We changed this in the text.
-
6.
In the last part of section 4, in the “neuroAIDS” section, citations are needed to document the connection and data indicating that ATP levels rise in the brain and that this is tied to cognitive impairment.
Answer 5: We added the reference below in the text.
Luis B. Tovar-y-Romo & Dennis L. Kolson & Veera Venkata Ratnam Bandaru & Julia L. Drewes & David R. Graham & Norman J. Haughey. Adenosine Triphosphate Released from HIV-Infected Macrophages Regulates Glutamatergic Tone and Dendritic Spine Density on Neurons. J Neuroimmune Pharmacol. DOI 10.1007/s11481-013-9471-7.
-
7.
Recent papers should be mentioned:
PMID:24842759, PMID:24158495 and especially: Idzko M, Ferrari D, Eltzschig HK., Nature. 2014 May 15;509(7500):310–7. doi: 10.1038/nature13085. PMID:24828189
Answer 6: We commented and added the reference above in the text.
Quality of written English: Not suitable for publication unless extensively edited.
Answer 7: We sent the paper to American Journal Experts. The certificate is attached.
Second round
Reviewer’s report
Reviewer 1: Neil S. Greenspan, CaseWestern Reserve University, United States of America
Comments to Authors:
Pacheco et al. have responded substantively to the previous reviews by tightening the focus of the manuscript to emphasize the role of purinergic receptors in the pathogenesis of HIV-1 infection of humans. The writing has also been substantially improved, although there are still a small number of passages requiring revision. The following comments note areas of the text requiring author responses.
-
1.
In the abstract, the last two sentences are in need of editing.
“Three recent reports have examined the relationship between the level of extracellular ATP, the mechanisms underlying purinergic receptors participating of in the infection mechanism of HIV-1 in the cell. Although preliminary, these results indicate that purinergic receptors are putative pharmacological targets to that should be further explored in future studies.”
-
2.
The second-to-last sentence at the end of the first paragraph continuing onto the top of page 4 appears to make more sense if “Although” and “it” are deleted. “Although The detailed signaling process that is triggered by the activation of most P2XRs, it has yet to be completely elucidated.”
-
3.
On the top of page 5, in the sentence beginning “A large body of evidence …,” the word “responses” might better be replaced by “phenomena,” as immune “responses” are most typically referred to by immunologists to indicate specifically coordinated events involving the proliferation and differentiation of lymphocytes and not separately for every sort of molecular or cellular mechanism that arises in the course of an immune response.
-
4.
The mediator adenosine is referred to as a “danger” molecule. While I recognize that this usage is widespread, I would advise not using it especially when, as here, “danger” is not defined. Frequently, molecules that are taken to correspond to “danger signals,” with minimal to no justification are in fact, in some circumstances, causes of danger. For example, I just saw a report that the massive tissue damage that characterizes Ebola virus infection is largely attributable not to direct virus-mediated effects but to the host response, in particular so-called cytokine storm (http://www.npr.org/blogs/goatsandsoda/2014/08/26/342451672/howebola-kills-you-its-not-the-virus) from my perspective, calling the release of adenosine a danger signal adds no insight not gleaned from a careful delineation of the effects of adenosine on various receptors and signaling pathways and can easily lead to incorrect conclusions and sloppy thinking.
-
5.
On page 9, in the first full paragraph, it is stated that about HIV-1 infection that: “viruses in the blood, which remains measurable throughout the course of infection …” The next paragraph seemingly contradicts that assertion (“…the reduction of plasma HIV-1 levels below the level of detection of commercially available tests and limited immune reconstitution (139–142).”).
-
6.
On page 13, I would add one word to the sentence quoted below, “Accordingly, recent reports of the role of purinergic receptors in the immunopathogenesis of HIV-1 infection indicate that they are putative pharmacological targets that should be further explored”.
Quality of written English: Acceptable
Authors’ response
We accept all of the reviewer’s suggestions. The text has been changed to accommodate them.
Reviewer’s report by Rachel Gerstein
University of Massachusetts Medical School, United States of America.
This reviewer provided no comments for publication.
References
Dubyak GR: Signal transduction by P2-purinergic receptors for extracellular ATP. Am J Respir Cell Mol Biol. 1991, 4 (4): 295-300.
Burnstock G, Williams M: P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000, 295 (3): 862-869.
Burnstock G: A basis for distinguishing two types of purinergic receptor. Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach. Edited by: Straub RW, Bolis L. 1978, New York: Raven, 107-118.
Ralevic V, Burnstock G: Receptors for purines and pyrimidines. Pharmacol Rev. 1998, 50 (3): 413-492.
Surprenant A, North RA: Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009, 71: 333-359.
Burnstock G, Fredholm BB, North RA, Verkhratsky A: The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf). 2010, 199 (2): 93-147.
Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M: Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994, 46 (2): 143-156.
Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Muller CE: International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev. 2011, 63 (1): 1-34.
Junger WG: Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011, 11 (3): 201-212.
Burnstock G, Kennedy C: Is there a basis for distinguishing two types of P2-purinoceptor?. Gen Pharmacol. 1985, 16 (5): 433-440.
Abbracchio MP, Burnstock G: Purinoceptors: are there families of P2X and P2Y purinoceptors?. Pharmacol Ther. 1994, 64 (3): 445-475.
Jacobson KA, Boeynaems JM: P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010, 15 (13–14): 570-578.
Rayah A, Kanellopoulos JM, Di Virgilio F: P2 receptors and immunity. Microbes and Infection/Institut Pasteur. 2012, 14 (14): 1254-1262.
Burnstock G: Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012, 34 (3): 218-225.
Khakh BS: Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001, 2 (3): 165-174.
North RA: Molecular physiology of P2X receptors. Physiol Rev. 2002, 82 (4): 1013-1067.
Jarvis MF, Khakh BS: ATP-gated P2X cation-channels. Neuropharmacology. 2009, 56 (1): 208-215.
Khakh BS, Bao XR, Labarca C, Lester HA: Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999, 2 (4): 322-330.
Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A: Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999, 2 (4): 315-321.
Khakh BS, Lester HA: Dynamic selectivity filters in ion channels. Neuron. 1999, 23 (4): 653-658.
Rettinger J, Schmalzing G: Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol. 2003, 121 (5): 451-461.
Pratt EB, Brink TS, Bergson P, Voigt MM, Cook SP: Use-dependent inhibition of P2X3 receptors by nanomolar agonist. J Neurosci. 2005, 25 (32): 7359-7365.
Sokolova E, Skorinkin A, Moiseev I, Agrachev A, Nistri A, Giniatullin R: Experimental and modeling studies of desensitization of P2X3 receptors. Mol Pharmacol. 2006, 70 (1): 373-382.
Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G: P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998, 17 (11): 3016-3028.
Erb L, Liao Z, Seye CI, Weisman GA: P2 receptors: intracellular signaling. Pflugers Arch. 2006, 452 (5): 552-562.
Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS: Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011, 63 (3): 641-683.
Faria RX, de Farias FP, Alves LA: Are second messengers crucial for opening the pore associated with P2X7 receptor?. Am J Physiol Cell Physiol. 2005, 288 (2): C260-C271.
Boarder MR, Weisman GA, Turner JT, Wilkinson GF: G protein-coupled P2 purinoceptors: from molecular biology to functional responses. Trends Pharmacol Sci. 1995, 16 (4): 133-139.
Barnard EA: The transmitter-gated channels: a range of receptor types and structures. Trends Pharmacol Sci. 1996, 17 (9): 305-309.
von Kügelgen I, Wetter A: Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000, 362 (4–5): 310-323.
von Kügelgen I: Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006, 110 (3): 415-432.
Burnstock G: Purine and pyrimidine receptors. Cell Mol Life Sci. 2007, 64 (12): 1471-1483.
Burnstock G, Knight GE: Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004, 240: 31-304.
Nihei OK, de Carvalho AC, Savino W, Alves LA: Pharmacologic properties of P(2Z)/P2X(7)receptor characterized in murine dendritic cells: role on the induction of apoptosis. Blood. 2000, 96 (3): 996-1005.
Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J, Chiozzi P, Di Virgilio F, Luttmann W: P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000, 486 (3): 217-224.
Di Virgilio F, Borea PA, Illes P: P2 receptors meet the immune system. Trends Pharmacol Sci. 2001, 22 (1): 5-7.
Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M: P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004, 10 (8): 821-827.
Lee DH, Park KS, Kong ID, Kim JW, Han BG: Expression of P2 receptors in human B cells and Epstein-Barr virus-transformed lymphoblastoid cell lines. BMC Immunol. 2006, 7: 22-
Linden J: Regulation of Leukocyte Function by Adenosine Receptors. Advances in Pharmacology. Volume 61. Edited by: Kenneth AJ, Joel L. 2011, Kansas City: Academic Press, 95-114.
Mei L, Du W, Gao W, Mei QB: Purinergic signaling: a novel mechanism in immune surveillance. Acta Pharmacol Sin. 2010, 31 (9): 1149-1153.
Idzko M, Dichmann S, Ferrari D, Di Virgilio F, la Sala A, Girolomoni G, Panther E, Norgauer J: Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002, 100 (3): 925-932.
Linden J: Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001, 41: 775-787.
Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J: International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001, 53 (4): 527-552.
Hasko G, Linden J, Cronstein B, Pacher P: Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008, 7 (9): 759-770.
Thiel M, Chambers JD, Chouker A, Fischer S, Zourelidis C, Bardenheuer HJ, Peter K: Effect of adenosine on the expression of b2-integrins and l-selectin of polymorphonuclear leukocytes in vitro. J Leukocyte Biol. 1996, 59: 671-682.
Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G: Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992, 148: 2201-2206.
Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R: Adenosine: a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985, 2: 1366-1371.
Richter J: Effect of adenosine analogues and cAMP-raising agents on TNF-, GM-CSF-, and chemotactic peptide-induced degranulation in single adherent neutrophils. J Leukocyte Biol. 1992, 51: 270-275.
Thiel M, Chouker A: Acting via A2 receptors, adenosine inhibits the production of tumor necrosis factor-a of endotoxin-stimulated human polymorphonuclear leukocytes. J Lab Clin Med. 1995, 124: 275-282.
Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M: The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2-generation, respectively. J Clin Invest. 1990, 85: 1150-1157.
Broussas M, Cornillet-Lefèbvre P, Potron G, Nguyen P: Inhibition of fMLP-triggered respiratory burst of human monocytes by adenosine: involvement of A3 adenosine receptor. J Leukocyte Biol. 1999, 66: 495-501.
Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS: Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol. 1996, 156: 3435-3442.
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS: Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009, 461 (7261): 282-286.
Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG: ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science (New York, NY). 2006, 314 (5806): 1792-1795.
Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schön P, Schwab A, Hanley PJ: Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010, 3 (132): ra55-ra-
McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P: Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010, 330 (6002): 362-366.
Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S: Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001, 21 (6): 1975-1982.
Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS: Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010, 467 (7317): 863-867.
Sluyter R, Barden JA, Wiley JS: Detection of P2X purinergic receptors on human B lymphocytes. Cell Tissue Res. 2001, 304 (2): 231-236.
Sakowicz-Burkiewicz M, Kocbuch K, Grden M, Szutowicz A, Pawelczyk T: Adenosine 5′-triphosphate is the predominant source of peripheral adenosine in human B lymphoblasts. J Physiol Pharmacol. 2010, 61 (4): 491-499.
Padeh S, Cohen A, Roifman CM: ATP-induced activation of human B lymphocytes via P2-purinoceptors. J Immunol. 1991, 146 (5): 1626-1632.
Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, Di Virgilio F: Increased proliferation rate of lymphoid cells transfected with the P2X7 ATP receptor. J Biol Chem. 1999, 274: 33206-33208.
Chvatchko Y, Valera S, Aubry JP, Renno T, Buell G, Bonnefoy JY: The involvement of an ATP-gated ion channel, P(2X1), in thymocyte apoptosis. Immunity. 1996, 5 (3): 275-283.
Apasov SG, Koshiba M, Chused TM, Sitkovsky MV: Effects of extracellular ATP and adenosine on different thymocyte subsets: possible role of ATP-gated channels and G protein-coupled purinergic receptor. J Immunol. 1997, 158 (11): 5095-5105.
Freedman BD, Liu QH, Gaulton G, Kotlikoff MI, Hescheler J, Fleischmann BK: ATP-evoked Ca2+ transients and currents in murine thymocytes: possible role for P2X receptors in death by neglect. Eur J Immunol. 1999, 29 (5): 1635-1646.
Nagy PV, Feher T, Morga S, Matko J: Apoptosis of murine thymocytes induced by extracellular ATP is dose- and cytosolic pH-dependent. Immunol Lett. 2000, 72 (1): 23-30.
Auger R, Motta I, Benihoud K, Ojcius DM, Kanellopoulos JM: A role for mitogen-activated protein kinase(Erk1/2) activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J Biol Chem. 2005, 280 (30): 28142-28151.
Lépine S, Le Stunff H, Lakatos B, Sulpice JC, Giraud F: ATP-induced apoptosis of thymocytes is mediated by activation of P2X7 receptor and involves de novo ceramide synthesis and mitochondria. Biochim Biophys Acta. 2006, 1761 (1): 73-82.
Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, Degawa M: P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol. 2006, 177 (5): 2842-2850.
Ross PE, Ehring GR, Cahalan MD: Dynamics of ATP-induced calcium signaling in single mouse thymocytes. J Cell Biol. 1997, 138 (5): 987-998.
Frascoli M, Marcandalli J, Schenk U, Grassi F: Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral gammadelta cells. J Immunol. 2012, 189 (1): 174-180.
Loomis WH, Namiki S, Ostrom RS, Insel PA: Hypertonic stress increases T cells interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem. 2003, 278: 4590-4596.
Yip L, Cheung CW, Corriden R, Chen Y, Insel PA, Junger WG: Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock. 2007, 27 (3): 242-250.
Corriden R, Insel PA, Junger WG: A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am J Physiol Cell Physiol. 2007, 293 (4): C1420-C1425.
Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG: Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009, 23 (6): 1685-1693.
Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, Junger WG: Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010, 88 (6): 1181-1189.
Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG: Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010, 116 (18): 3475-3484.
Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F: Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008, 1 (39): ra6-
Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S: Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010, 285 (23): 17406-17416.
Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E, Rubini M, Di Virgilio F: An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996, 87 (2): 682-690.
Filippini A, Taffs RE, Sitkovsky MV: Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci U S A. 1990, 87 (21): 8267-8271.
Langston HP, Ke Y, Gewirtz AT, Dombrowski KE, Kapp JA: Secretion of IL-2 and IFN-gamma, but not IL-4, by antigen-specific T cells requires extracellular ATP. J Immunol. 2003, 170 (6): 2962-2970.
Manohar M, Hirsh MI, Chen Y, Woehrle T, Karande AA, Junger WG: ATP release and autocrine signaling through P2X4 receptors regulate γδ T cell activation. J Leukoc Biol. 2012, 92 (4): 787-794.
Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F: ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011, 4 (162): ra12-
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F: The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006, 176 (7): 3877-3883.
Volonte C, Apolloni S, Skaper SD, Burnstock G: P2X7 receptors: channels, pores and more. CNS Neurol Disord Drug Targets. 2012, 11 (6): 705-721.
Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA: IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010, 6 (2): e1000661-
Trautmann A: Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009, 2 (56): pe6-pe-
Zimmermann H, Zebisch M, Strater N: Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8 (3): 437-502.
Idzko M, Ferrari D, Eltzschig HK: Nucleotide signalling during inflammation. Nature. 2014, 509 (7500): 310-317.
Gorini S, Gatta L, Pontecorvo L, Vitiello L, la Sala A: Regulation of innate immunity by extracellular nucleotides. Am J Blood Res. 2013, 3 (1): 14-28.
Corriden R, Insel PA: Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010, 3 (104): re1-
Di Virgilio F: Purinergic signalling in the immune system. A brief update. Purinergic Signal. 2007, 3 (1–2): 1-3.
Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM: The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood. 2012, 120 (12): 2365-2375.
Dubyak GR: P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012, 14 (11): 1697-1706.
Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, Smith NC: The role of the P2X(7) receptor in infectious diseases. PLoS Pathog. 2011, 7 (11): e1002212-
Molloy A, Laochumroonvorapong P, Kaplan G: Apoptosis, but not necrosis, of infected monocytes is copled with killing of intracellular bacillus Calmette-Guerin. J exp Med. 1994, 180 (4): 1499-1509.
Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS: ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997, 7 (3): 433-444.
Kusner DJ, Barton JA: ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J Immunol. 2001, 167 (6): 3308-3315.
Stober CB, Lammas DA, Li CM, Kumararatne DS, Lightman SL, McArdle CA: ATP-mediated killing of Mycobacterium bovis bacille Calmette-Guérin within human macrophages is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J Immunol. 2001, 166 (10): 6276-6286.
Smith RA, Alvarez AJ, Estes DM: The P2X7 purinergic receptor on bovine macrophages mediates mycobacterial death. Vet Immunol Immunopathol. 2001, 78 (3–4): 249-262.
Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA: ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol. 2001, 167 (6): 3300-3307.
Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS: A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol. 2003, 171 (10): 5442-5446.
Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, Wiley JS, Britton WJ: A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. AmJ RespirCrit Care Med. 2007, 175 (4): 360-366.
Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS: A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006, 281 (4): 2079-2086.
Franco-Martínez S, Niño-Moreno P, Bernal-Silva S, Baranda L, Rocha-Meza M, Portales-Cervantes L, Layseca-Espinosa E, González-Amaro R, Portales-Pérez D: Expression and function of the purinergic receptor P2X7 in patients with pulmonary tuberculosis. Clin Exp Immunol. 2006, 146 (2): 253-261.
Placido R, Auricchio G, Falzoni S, Battistini L, Colizzi V, Brunetti E, Di Virgilio F, Mancino G: P2X(7) purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cellular immunology. 2006, 244 (1): 10-18.
Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M, Lammas DA: ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunol. 2008, 9: 35-
Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM: Modulation of P2Z/P2X(7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001, 280 (1): C81-9.
Coutinho-Silva R, Stahl L, Raymond MN, Jungas T, Verbeke P, Burnstock G, Darville T, Ojcius DM: Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003, 19 (3): 403-412.
Darville T, Welter-Stahl L, Cruz C, Sater AA, Andrews CW, Ojcius DM: Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol. 2007, 179 (6): 3707-3714.
Kolli BK, Kostal J, Zaborina O, Chakrabarty AM, Chang K: Leishmania-released nucleoside diphosphate kinase prevents ATP-mediated cytolysis of macrophages. Mol Biochem Parasitol. 2008, 158 (2): 163-175.
Chaves SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B: Modulation of P2X(7) purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009, 11 (10–11): 842-849.
Marques-da-Silva C, Chaves MM, Rodrigues JC, Corte-Real S, Coutinho-Silva R, Persechini PM: Differential modulation of ATP-induced P2X7-associated permeabilities to cations and anions of macrophages by infection with Leishmania amazonensis. PLoS One. 2011, 6 (9): e25356-
Marques-da-Silva C, Chaves MM, Chaves SP, Figliuolo VR, Meyer-Fernandes JR, Corte-Real S, Lameu C, Ulrich H, Ojcius DM, Rossi-Bergmann B, Coutinho-Silva R: Infection with Leishmania amazonensis upregulates purinergic receptor expression and induces host-cell susceptibility to UTP-mediated apoptosis. Cell Microbiol. 2011, 13 (9): 1410-1428.
Correa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R: Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010, 12 (6): 497-504.
Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC, Jamieson SE, Blackwell JM, Wiley JS, Smith NC: P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010, 184 (12): 7040-7046.
Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix LA, Mui EJ, Boulter NR, Miller EN, Fuller SJ, Wiley JS, Castellucci L, Boyer K, Peixe RG, Kirisits MJ, Elias Lde S, Coyne JJ, Correa-Oliveira R, Sautter M, Smith NC, Lees MP, Swisher CN, Heydemann P, Noble AG, Patel D, Bardo D, Burrowes D, McLone D, Roizen N, Withers S, Bahia-Oliveira LM, McLeod R, et al: Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010, 11 (5): 374-383.
Miller CM, Zakrzewski AM, Ikin RJ, Boulter NR, Katrib M, Lees MP, Fuller SJ, Wiley JS, Smith NC: Dysregulation of the inflammatory response to the parasite Toxoplasma gondii in P2X7 receptor-deficient mice. Int J Parasitol. 2011, 41 (3–4): 301-308.
Petcu DJ, Aldrich CE, Coates L, Taylor JM, Mason WS: Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology. 1988, 167 (2): 385-392.
Schulze A, Gripon P, Urban S: Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007, 46 (6): 1759-1768.
Taylor JM, Han Z: Purinergic receptor functionality is necessary for infection of human hepatocytes by hepatitis delta virus and hepatitis B virus. PLoS One. 2010, 5 (12): e15784-
Croon JJ, Wolff HL: The inhibition of yellow fever virus multiplication by suramin: a preliminary note. Acta Leiden. 1982, 48: 5-8.
Xu K, Ren H, Zhu J, Yang Y, Liao F: Suramin inhibits the in vitro expression of encephalitis B virus proteins NS3 and E. J Huazhong Univ Sci Technolog Med Sci. 2003, 23 (4): 375-379.
Zandberg M, van Son WJ, Harmsen MC, Bakker WW: Infection of human endothelium in vitro by cytomegalovirus causes enhanced expression of purinergic receptors: a potential virus escape mechanism?. Transplantation. 2007, 84 (10): 1343-1347.
Levy JA: Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993, 57 (1): 183-289.
Piatak M, Saag MS, Yang LC, Clark SJ, Kappes JC, Luk KC, Hahn BH, Shaw GM, Lifson JD: High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993, 259 (5102): 1749-1754.
Fauci AS: Host factors and the pathogenesis of HIV-induced disease. Nature. 1996, 384 (6609): 529-534.
Freed EO: HIV-1 replication. Somat Cell Mol Genet. 2001, 26 (1–6): 13-33.
Costin JM: Cytopathic mechanisms of HIV-1. Virol J. 2007, 4: 100-
Lieberman J, Shankar P, Manjunath N, Andersson J: Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001, 98 (6): 1667-1677.
Peretz Y, Cameron C, Sekaly RP: Dissecting the HIV-specific immune response: a systems biology approach. Curr Opin HIV AIDS. 2012, 7 (1): 17-23.
Ries M, Pritschet K, Schmidt B: Blocking type I interferon production: a new therapeutic option to reduce the HIV-1-induced immune activation. Clin Dev Immunol. 2012, 2012: 534929-
Benecke A, Gale M, Katze MG: Dynamics of innate immunity are key to chronic immune activation in AIDS. Curr Opin HIV AIDS. 2012, 7 (1): 79-85.
Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF: The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004, 10 (11): 525-531.
Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J: Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997, 277 (5322): 112-116.
Cohen OJ, Fauci AS: Benchmarks for antiretroviral therapy. J Clin Invest. 2000, 105 (6): 709-710.
Bailey J, Blankson JN, Wind-Rotolo M, Siliciano RF: Mechanisms of HIV-1 escape from immune responses and antiretroviral drugs. Curr Opin Immunol. 2004, 16 (4): 470-476.
Corbeau P, Reynes J: Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011, 117 (21): 5582-5590.
Sension MG: Long-Term suppression of HIV infection: benefits and limitations of current treatment options. J Assoc Nurses AIDS Care. 2007, 18 (1 Suppl): S2-S10.
De Clercq E: New developments in anti-HIV chemotherapy. Biochim Biophys Acta. 2002, 1587 (2–3): 258-275.
Simon V, Vanderhoeven J, Hurley A, Ramratnam B, Louie M, Dawson K, Parkin N, Boden D, Markowitz M: Evolving patterns of HIV-1 resistance to antiretroviral agents in newly infected individuals. AIDS. 2002, 16 (11): 1511-1519.
Jiang Y, Liu X, De Clercq E: New therapeutic approaches targeted at the late stages of the HIV-1 replication cycle. Curr Med Chem. 2011, 18 (1): 16-28.
Seror C, Melki MT, Subra F, Raza SQ, Bras M, Saidi H, Nardacci R, Voisin L, Paoletti A, Law F, Martins I, Amendola A, Abdul-Sater AA, Ciccosanti F, Delelis O, Niedergang F, Thierry S, Said-Sadier N, Lamaze C, Métivier D, Estaquier J, Fimia GM, Falasca L, Casetti R, Modjtahedi N, Kanellopoulos J, Mouscadet JF, Ojcius DM, Piacentini M, Gougeon ML, et al: Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med. 2011, 208 (9): 1823-1834.
Schuitemaker H, Kootstra NA, de Goede RE, de Wolf F, Miedema F, Tersmette M: Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991, 65 (1): 356-363.
Coiras M, Lopez-Huertas MR, Perez-Olmeda M, Alcami J: Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009, 7 (11): 798-812.
Duncan CJ, Sattentau QJ: Viral determinants of HIV-1 macrophage tropism. Viruses. 2011, 3 (11): 2255-2279.
Hazleton JE, Berman JW, Eugenin EA: Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol. 2012, 188 (9): 4488-4495.
Swartz TH, Esposito AM, Durham ND, Hartmann BM, Chen BK: P2X-Selective Purinergic Antagonists Are Strong Inhibitors of HIV-1 Fusion during both Cell-to-Cell and Cell-Free Infection. J Virol. 2014, 88 (19): 11504-11515.
Tovar-Y-Romo LB, Kolson DL, Bandaru VV, Drewes JL, Graham DR, Haughey NJ: Adenosine triphosphate released from HIV-infected macrophages regulates glutamatergic tone and dendritic spine density on neurons. J Neuroimmune Pharmacol. 2013, 8: 998-1009.
Sorrell ME, Hauser KF: Ligand-gated purinergic receptors regulate HIV-1 Tat and morphine related neurotoxicity in primary mouse striatal neuron-glia co-cultures. J Neuroimmune Pharmacol. 2014, 9 (2): 233-244.
Pingle SC, Jajoo S, Mukherjea D, Sniderhan LF, Jhaveri KA, Marcuzzi A, Rybak LP, Maggirwar SB, Ramkumar V: Activation of the adenosine A1 receptor inhibits HIV-1 tat-induced apoptosis by reducing nuclear factor-kappaB activation and inducible nitric-oxide synthase. Mol Pharmacol. 2007, 72 (4): 856-867.
Fotheringham J, Mayne M, Holden C, Nath A, Geiger JD: Adenosine receptors control HIV-1 Tat-induced inflammatory responses through protein phosphatase. Virology. 2004, 327 (2): 186-195.
Kumar V, Sharma A: Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009, 616 (1–3): 7-15.
By Y, Durand-Gorde JM, Condo J, Lejeune PJ, Fenouillet E, Guieu R, Ruf J: Monoclonal antibody-assisted stimulation of adenosine A2A receptors induces simultaneous downregulation of CXCR4 and CCR5 on CD4+ T-cells. Hum Immunol. 2010, 71 (11): 1073-1076.
Prevelige PE: New approaches for antiviral targeting of HIV assembly. J Mol Biol. 2011, 410 (4): 634-640.
Hayes MM, Lane BR, King SR, Markovitz DM, Coffey MJ: Prostaglandin E(2) inhibits replication of HIV-1 in macrophages through activation of protein kinase A. Cell Immunol. 2002, 215 (1): 61-71.
Barbera-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, Pelegrin P: P2X7 receptor-stimulation causes fever via PGE2 and IL-1beta release. FASEB J. 2012, 26 (7): 2951-2962.
Leal DB, Streher CA, Bertoncheli Cde M, Carli LF, Leal CA, da Silva JE, Morsch VM, Schetinger MR: HIV infection is associated with increased NTPDase activity that correlates with CD39-positive lymphocytes. Biochim Biophys Acta. 2005, 1746 (2): 129-134.
Acknowledgements
The National Counsel of Technological and Scientific Development (CNPq), Foundation for Research Support of the State of Rio de Janeiro (Faperj) and Oswaldo Cruz Institute supported this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PAFP wrote the main body of the review and LGBF reviewed and formatted the structure of the text. RXF has reviewed the topics about purinergic receptor and ICNPP has reviewed topics related to HIV-1 infection. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pacheco, P.A., Faria, R.X., Ferreira, L.G. et al. Putative roles of purinergic signaling in human immunodeficiency virus-1 infection. Biol Direct 9, 21 (2014). https://doi.org/10.1186/1745-6150-9-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1745-6150-9-21