Abstract
Background
Toxoplasmosis is a zoonosis caused by an obligate intracellular parasite, Toxoplasma gondii, which affects warm-blooded animals including humans. Its prevalence rates usually vary in different regions of the planet.
Methods
In this study, an analysis of the seroprevalence of toxoplasmosis among Brazilian students was proposed by means of IgG specific antibodies detection. The presence of anti-Toxoplasma gondii antibodies by indirect fluorescent antibody test (IFAT) was also evaluated in order to compare it with enzyme-linked immunosorbent assay (ELISA) and to assess the use of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) and o-phenylenediamine dihydrochloride chromogens.
Results
The IFAT method showed a seroprevalence of 22.3%. These results were similar to those obtained by ELISA (24.1%). The seroprevalence was directly estimated from the IgG avidity, which showed that in a sample of 112 students, three of them had acute infection, an incidence of 1.6% in the studied population.
Conclusion
In this study, the use of different chromogenic substrates in immunoenzymatic ELISA assays did not display different sensitivity in the detection of T. gondii-reagent serum. The extrapolation of results to this population must be carefully considered, since the investigation was conducted on a reduced sample. However, it allows us to emphasize the importance of careful and well prepared studies to identify risk factors for toxoplasmosis, to adopt preventive measures and to offer guidance to at-risk populations about the disease.
Similar content being viewed by others
Background
Toxoplasma gondii is a protozoan parasite that is responsible for toxoplasmosis in warm-blooded animals [1–7]. The infection is spread mainly through contact with oocysts eliminated in feces of infected cats, ingestion of contaminated raw or undercooked meat and congenital infection [1, 8–14]. The environmental contamination by this protozoa demands attention since it may trigger other routs of transmission and can lead to outbreaks, such as the waterborne epidemic in Santa Isabel do Ivaí, Paraná state, Brazil (23° 0′ 19″ S, 53° 11′ 16″ W) [15].
Human infection is usually asymptomatic. The main clinical signs after the onset of the disease include lymphadenitis, fever, asteny, and myalgia. Encephalitis, meningoencephalitis, ocular lesions, septic syndrome, myocarditis or hepatitis may be found occasionally [1, 3, 16, 17]. Symptoms are manifested mainly in immunocompromised people and newborns [18–21].
Recently the diagnosis of toxoplasmosis has been drawing close attention. Several serological techniques have been applied and have shown good sensitivity, specificity, and are quickly carried out. Among different diagnostic techniques, the following are noteworthy: Sabin and Feldman technique, indirect fluorescent antibody test (IFAT), complement fixation (CF), hemagglutination (HA), enzyme-linked immunosorbent assay (ELISA) and immunosorbent agglutination assay (ISAGA) [22–27].
Seroprevalence indexes have varied in different countries and regions of the planet [28]. Serological research showed rates of 6.7% in Korea, 12.3% in China, 22.5%, in the US adult population, 23.9% in Nigeria, 38.8% in Spain, 46% in Tanzania and 47% in rural zones of the France [29–35]. A study with anti-T. gondii antibodies carried out in the Republic of Benin revealed a seroprevalence of 87.7% [36]. In different regions in Brazil, the seropositive rate varied between 37% and 91% [37, 4].
Three suspicious cases of toxoplasmosis were reported in São Paulo State University (UNESP), in Assis, São Paulo state, Brazil (22° 39′ 42″ S, 50° 24′ 44″ W) stirring up a series of controversies and discussions. The first one refers to clinical likelihood of the disease in college students. However, no serological test was conducted to uphold the diagnosis. The second one is the relation between these cases was the increased cat population. And, finally, a student who had an eye affliction and tested positive for T. gondii.
Since these reports and suppositions were based on inconsistent diagnostic assertions and subjective causal links, without the due clinical evaluation and laboratory diagnostic evidence, a project was devised to find out the seroprevalence and incidence of toxoplasmosis in a sample of UNESP students, by means of serological tests for IgG specific antibodies detection. In addition, the study also verified the presence of anti-T. gondii antibodies in students by IFAT, in order to compare IFAT and ELISA techniques for the toxoplasmosis diagnosis, and to evaluate the use of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) and o-phenylenediamine dihydrochloride (OPD) chromogens in the ELISA test for the toxoplasmosis diagnosis.
Methods
The project was carried out at the Laboratory of Human Parasitology and Immunology of São Paulo State University (UNESP), in Assis, with the cooperation of the Laboratory of Zoonoses and Public Health of the Department of Preventive Veterinary Medicine, State University of Londrina (UEL), in Londrina, Paraná state, Brazil (23° 19′ 39.5″ S, 51° 11′ 56.5″ W) and of the Laboratory of Protozoonoses of the Institute of Tropical Medicine of São Paulo, University of São Paulo (USP), São Paulo, São Paulo state, Brazil (23° 33′ 18.5″ S, 46° 40′ 18.2″ W).
Characteristics of the area and population under study
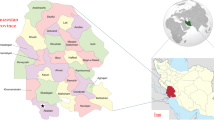
Assis is located in western São Paulo state, Brazil, 434 km away from the state capital and comprises a population of 100.911 inhabitants. The annual average temperature is 21.37°C (Figure 1) [38].
Assis is located in the west region of São Paulo state, Brazil, 434 km away from the state capital and comprises a population of 100.911 inhabitants [38]. The annual average temperature is 21.37°C.
Serological tests were carried out in 112 UNESP freshmen of Assis campus, within an age range from 17 to 25 years old. The selection of students was random (by a raffle) and the sample size was proportional to the number of students in each course (Table 1). Freshman students answered a questionnaire, in order to establish the likelihood of epidemiological correlations, risk factors and contact with cats before being accepted into the university.
Collection of blood and serum samples
The serological information of the students was recorded in individual files and kept confidential. Two blood samples were collected from each voluntary subject of the research. The first one was immediately after the students arrived for the beginning of the 2009 school year and the second one, six months after the first sample had been collected. A 5-mL blood sample was collected from each student, by brachial venous puncture. Meticulous aseptic cautions were taken for blood collection and for the protection of those in charge of it. The samples were sent to the Laboratory of Human Parasitology and Immunology for blood processing, plasma separation and storage for later serological analysis methods. Each serum sample was obtained from the whole blood, after centrifugation at 350 × g, for 15 minutes. The resulting supernatant was stored at −20°C.
Indirect Fluorescent Antibody Test (IFAT)
The indirect fluorescent antibody test (IFAT) technique for the screening of anti-T. gondii antibodies, of the IgG class, was carried out according to the methodology described by Camargo [23]. Human anti-IgG serum produced from rabbit inoculations was employed conjugated with fluorescein isothiocyanate (Sigma Chemical Corp., USA). For each serum sample, dilutions at 1:16, 1:64, 1:256, 1:1024 and 1:14096 were prepared. All reactions had a previously known positive and negative control serum. The reading of the plates was made in a fluorescence microscope (Nikon™, Japan). Dilutions greater than or equal to 1:16A were considered positive results.
ELISA immunoenzymatic test
Anti-T. gondii IgG antibodies were determined by the methodology of Camargo [24] and Uchôa et al. [39]. Plates with flat-bottom wells (Corning®, USA) sensitized with 1 μg/mL of T. gondii soluble antigen extract were used for OPD ELISA; and those sensitized with 10 μg/mL of T. gondii soluble antigen extract were used for ABTS ELISA. Plates were sensitized with antigen by incubation at 4°C for 18 hours, with 100 μL of antigen solution per well. After washing, plates were blocked by incubation for one hour at 37°C with 250 μL of PBS-T per cavity (0.05% of Tween 20 in phosphate-buffered saline solution – PBS) containing 3 g of skimmed milk powder (Molico®, Nestlé, Brazil). Afterward, plates were washed five times in PBS-T for five-minute periods. Plates containing serum with human-peroxydase-conjugated anti-IgG dilutions (Sigma®, USA) were incubated for the test in stove for one hour. Following both incubations, plates were washed three times in PBS-T for five minutes. The color reaction was carried out subsequently in the dark for 15 minutes at room temperature with 100 μL of the OPD chromogenic solution (o-phenylenediamine 0.05%, Sigma® + citric acid 1% + Na2HPO4 1.45% in H2O, addition of 10 μL de H2O2 30% for each 20 mL of the solution) and was quenched by the addition of 25 μL of 2 M sulfuric acid solution. For the OPD chromogenic solution, the optical density (OD) was determined by the reading at 492 nm, and for the ABTS chromogenic solution, by the reading at 414 nm and 612 nm, in a plate spectrophotometer (700 Plus Spectrophotometer, Fendo, Brazil). For OPD ELISA and ABTS ELISA, in both the first analysis and the one six months after, the cutoff point was obtained from the mean of results from negative control sera evaluated by IFAT plus two standard deviations.
IgG avidity
The same procedure was used for IgG analysis through in-house ELISA, according to the methodology described by Hedman et al.[40]. The plate was coated by the addition, for 24 hours at 4°C, of 100 μL of T. gondii protein antigen to the wells at 1 μg/mL concentration, in an 8 M urea chaotropic (Sigma®) solution in PBS. After blocking possible free sites, the wells received 100 μL of PBS-T diluted serum samples (1/100, 1/200, 1/400, 1/800). Plates were incubated for one hour at 37°C and washed five times in PBS-T. For each dilution, two wells received additional ten-minute incubation at 37°C with 6 M urea neutral chaotropic solution (Sigma®) in PBS-T. Wells containing the control solutions were kept in PBS-T. Afterwards, the antibody development was carried out with the use of human anti-IgG peroxydase conjugate (Sigma®).
The estimation of effective titer of each serum was carried out by means of the isolated dilutions of each sample in a log-log linear regression model. The titers of total or high avidity chaotropic-resistant antibodies were achieved by means of log-log regression, with the use of the values able to generate 1.0 absorbance in ELISA (Log = 0). Avidity (AVT) was determined by the percentage of chaotropic resistant titers. Samples with AVT values higher than 30% were considered as high avidity. The optical density (OD) was determined by the reading at 492 nm in a plate spectrophotometer.
Statistical analysis
The correlation rate was used for the analysis among the interspecific titers and the analysis of association among the positive results and variables related to risk factors (gender, direct contact with soil, contact with cats, consumption of raw or undercooked meat and raw vegetables). Values were submitted to the chi-square analysis, with the help of the Epi Info version 6.04 statistics software [41]. A reliable 95% range was considered and p-value < 0.05 was considered statistically significant. In order to check the agreement among ELISA and OPD or ABTS chromogen and IFAT diagnosis tests, the results were reviewed by the kappa test (K), and by sensitivity, specificity, positive predictive value and negative predictive value. Statistical comparisons were made using the GraphPad Prism version 5.00 software for Windows (Graphpad Software, USA).
Ethics committee approval
The present study was approved by the Research Ethics Committee of Marília Medical School (FAMEMA – Marília, SP, Brazil) under protocol n. 449/08. It included only patients who authorized the collection of blood samples through free informed consent according to the Brazilian National Committee of Ethics in Research (CONEP), resolution 236/96, National Health Council, Brazilian Ministry of Health.
Results
Serum samples from 112 students were submitted to anti-T. gondii IgG antibody screening by means of IFAT and ELISA with OPD or ABTS chromogen substrate. The correlation between the results obtained in the immunoenzymatic and immunofluorescence assays may be seen in Table 2, in which two discordant results can be noted, two samples that tested positive by OPD ELISA were negative by IFAT whereas two other samples that were positive by ABTS ELISA tested negative by IFAT.
The comparison between OPD and ABTS ELISA enzymatic assays showed high agreement (kappa = 0.9024), 92.5% sensitivity (95% confidence interval – CI: 90.5-96.3) and 97.6% specificity (95% CI: 93.2-97.8) and the positive predictive values were 92.5% (95% CI: 90.6-96.3) and negative predictive values 97.6% (95% CI: 93.2-97.8) (Figure 2).
Quantitative reactivity and linear regression analysis of IgG anti-T. gondiiin 112 serum samples from university students using ELISA with chromogen substrate OPD and ABTS. Interruptions in the axes show the thresholds of positivity of the assays (ELISA OPD cutoff = 0.486 and ELISA ABTS cutoff = 0.239).
According to IFAT, the usual standard, the seroprevalence was 22.3% (95% CI: 14.5-28.7), similar to the one found in two imunoenzymatic tests. The OPD ELISA test showed a seroprevalence of 24.1% (95% CI: 16.2-32.0), the same found in the ABTS ELISA.
The confirmation and comparison of IgG serological test results by IFAT and ELISA with OPD and ABTS chromogen substrate methods are shown in Table 2. There is a high agreement among the three methods. The comparison of IFAT with OPD and ABTS ELISA test showed a higher agreement than the one found among the immunoenzymatic tests alone (kappa = 0.9499) with 100% of sensitivity (95% CI: 97.6%-100), specificity of 97.7% (95% CI: 94.2-99.1), positive predictive value of 92.5% (95% CI: 88.6-93.8), and negative predictive value of 100% (95% CI: 96.7-100).
The use of different chromogenic substrates in the immunoenzymatic ELISA assays did not show different sensitivity for detecting T. gondii-reagent serum. The ELISA test with OPD chromogenic substrate with plates sensitized with 1 μg of antigen revealed 27 positive sera, the same result observed in the ELISA IgG test with ABTS chromogenic substrate and plates sensitized with 10 μg of antigen.
After six months, new blood samples were examined, by means of IFAT and ELISA with OPD and ABTS chromogen substrates, in order to check the presence of IgG anti-T. gondii antibodies. The analyses of 112 students showed that three who tested negative in the first time, six months after were seropositive by IFAT, OPD ELISA and ABTS ELISA. The other serum samples showed the same results of first analysis, with exception of four samples: two were negative by IFAT and OPD ELISA and positive by ABTS ELISA whereas the other two were negative by IFAT and ABTS ELISA and positive by OPD ELISA. The three seroconverted samples were submitted to IgG avidity test. Students showed low IgG avidity, indicating recently acquired infection (Table 3).
In the present study, the seroprevalence of toxoplasmosis was 22.3% and 25% in the first and second evaluation, respectively. The incidence was directly estimated from the IgG avidity, which showed that, from the 112 students, three had results suggesting acute infection with an incidence of 1.6% (95% CI: 0.6-4.7%), which allows projecting an annual incidence of 3.2% in the studied population.
Table 4 shows the distributions of the variables according to T. gondii infection frequency. No differences were observed when variables taken into account were: gender (χ2 = 0.23, p > 0.05), contact with the cats of the university (χ2 = 1.4; p > 0.05), consumption of raw vegetables (χ2 = 0.11; p > 0.05), and the direct contact with soil (χ2 = 0.58, p > 0.05). The variables contact with cats outside the university (χ2 = 4.2; p < 0.05) and ingestion of raw or undercooked meat (χ2 = 5.0, p < 0.05) were the statistically significant.
Discussion
The screening of IgG anti-T. gondii antibodies by IFAT showed a 22.3% prevalence in students. The seroprevalence data achieved by IFAT serological method were confirmed by the immunoenzymatic ELISA assay with OPD and ABTS chromogenic substrate (24.1%). The tests had highly coherent results, an unusual fact for assays with different antigens such as IFAT and hemagglutination [24].
Therefore, as proposed by Tanyuksel et al.[42] and Reis et al.[43], the avidity test was employed to identify cases suggesting recent infection. The results enabled the projection of an annual seroconversion of 3.2%. Although the avidity test may help to explain the Toxoplasma gondii acute infection diagnosis, some patients have shown persistent low avidity antibodies against the lysate of T. gondii antigenic cells for several months [44, 45]. On the other hand, IgG antibody maturity may occur in different periods, and it will set an analytical and interpretive trend of the results [46, 47].
Another aspect that may interfere in seroprevalence results of a given sample refers to the chromogen used in immunoenzymatic assays. In addition, when comparing OPD chromogen sensitivity with ABTS, it is possible to find that OPD chromogen is more sensitive and detects antibodies expressing higher optical densities as previously reported [48]. Thus, we used a higher concentration of antigen to sensitize the plate in ELISA with ABTS due to lower detection sensitivity of this chromogen.
In this study, ELISA test with IgG antibodies, ABTS or OPD chromogen, demonstrated the same sensitivity and specificity for toxoplasmosis reagent sera (Table 2). However, it is important to emphasize that values could be the result of the amount of antigen employed in the sensitization of plates, since 10 μg was used in the ELISA test with ABTS chromogen and, in the ELISA test with OPD, only 1 μg was used. This difference in antigen amount may have favored the convergence of results of toxoplasmosis seroprevalence in immunoenzymatic assays.
A careful evaluation of serological tests may result in useful data for the prevention and institution of protocols for the detection of groups at risk, including the IgG avidity test. Transversal studies, as simple as this one, may help in a correct analysis of the results and, accordingly, collaborate for proposals and development of strategies to facilitate the interpretation of diagnosis, and this may result in more reasonable prophylactic measures for controlling this infection.
Soil and consumption of food contaminated with oocysts are considered, in some regions, the major sources of infection by T. gondii[49]. However, in the sample of the present study, the relation between these variables and the frequency of seropositive results was not statistically significant. On the other hand, the variable contact with cats outside the university possibly had a more important role, based on a higher frequency of seropositive results among those who had contact with those animals (76.0%) and those who were used to consume raw or undercooked meat (92.0%). Such differences are considered statistically significant, in agreement with previous studies, in which frequent ingestion of raw or undercooked meat and contact with oocysts of infected felines are considered the main risk factors for T. gondii infection [49–52].
Concerning gender, no significant differences were noted, which is in agreement with Souza [53], Daguer et al. [54] and Sharif et al. [55].
Conclusions
In the present study, the use of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) and O-phenylenediamine dihydrochloride (OPD) chromogens in immunoenzymatic ELISA assay with adequate quantities of T. gondii antigen for each substrate showed the same sensitivity for detection of toxoplasmosis reagent sera (Table 2).
According to the current findings, the cat population within the university is not a risk factor for T. gondii infection. Therefore, one may conclude that the most relevant risk factors for toxoplasmosis were contact with cats outside UNESP and eating raw or uncooked meat (Table 4).
The extrapolation of these results to the general population must be carefully considered, since the investigation was conducted on a reduced sample. However, it allows stating the importance of careful and well prepared studies, not only to identify risk factors for toxoplasmosis, but also to adopt preventive measures and offer guidance to at-risk populations. Thus, the implementation of simple cross-sectional studies may provide important epidemiological contribution and establish consistent causal associations, avoiding thereby the development of hypotheses that do not support rigorous questioning.
References
Tenter AM, Heckeroth AR, Weis LM: Toxoplasma gondii: from animals to humans.Int J Parasitol 2000,30(12–13):1217–58.
Marques JM, Da Silva DV, Correia NAB, Velásquez LG, Da Silva RC, Langoni H, et al.: Prevalence and risk factors for human toxoplasmosis in a rural community.J Venom Anim Toxins Incl Trop Dis 2008,14(4):673–84. [http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1678-91992008000400010] 10.1590/S1678-91992008000400010
Langoni H: Zoonoses and human beings.J Venom Anim Toxins Incl Trop Dis 2004,10(2):111. [http://www.scielo.br/scielo.php?pid=S1678-91992004000200001&script=sci_arttext]
Vasconcelos CGC: Occupational zoonoses: Serum-epidemiological survey of veterinary students and analysis of risk of Leptospirosis, Brucellosis and Toxoplasmosis.J Venom Anim Toxins Incl Trop Dis 2004,10(2):192. [http://www.jvat.org.br/full/j-2004/volume_10/number_2/thesis2_10_2_2004.htm]
Muraro LS, Caramori Júnior JG, Amendoeira MRR, Pereira JA, Filho JXO, Vicente RT, et al.: Seroprevalence ofToxoplasma gondiiinfection in swine matrices in Nova Mutum and Diamantino, Mato Grosso.Bras Rev Bras Parasitol Vet 2010,23(4):254–5.
Vilela SMO, Silva JSA, Pinheiro Junior JW, Moraes EPBX, Saukas TN, Gondim LFP, et al.: Sparrows (Passer domesticusL.) as intermediary hosts ofToxoplasma gondiiin poultry farms from the “agreste” region of Pernambuco, Brazil.Pesq Vet Bras 2011,31(2):169–72. 10.1590/S0100-736X2011000200013
Dubey JP, Lago EG, Gennari SM, Su C, Jones JL: Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology.Parasitology 2012,139(11):1375–424. 10.1017/S0031182012000765
Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ: Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water.N Engl J Med 1982,307(11):666–9. 10.1056/NEJM198209093071107
Coutinho SG, Lobo R, Dutra G: Isolation ofToxoplasmafrom the soil during an outbreak of toxoplasmosis in a rural area in Brazil.J Parasitol 1982,68(5):866–8. 10.2307/3280995
Dubey JP, Towle A (Eds): Toxoplasmosis in sheep: a review and annotated bibliography. St. Albans, England: Commonwealth Institute of Parasitology; 1986.
Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Oréfice F, Addiss DG: Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state.Bras Emerg Infect Dis 2003,9(1):55–62. 10.3201/eid0901.020160
Silva RC, Souza LC, Langoni H, Tanaka EM, Lima VY, Silva AV: Risk factors and presence of antibodies toToxoplasma gondiiin dogs from the coast of São Paulo State.Bras Pesq Vet Bras 2010,30(2):161–6. 10.1590/S0100-736X2010000200011
Hill D, Coss C, Dubey JP, Wroblewski K, Sautter M, Hosten T, et al.: Identification of a sporozoite-specific antigen fromToxoplasma gondii.J Parasitol 2011,97(2):328–37. 10.1645/GE-2782.1
Robert-Gangneux F, Dardé ML: Epidemiology of and diagnostic strategies for toxoplasmosis.Clin Microbiol Rev 2012,25(2):264–96. 10.1128/CMR.05013-11
de Moura L, Bahia-Oliveira LM, Wada MY, Jones JL, Tuboi SH, Carmo EH, et al.: Waterborne toxoplasmosis, Brazil, from field to gene.Emerg Infect Dis 2006,12(2):326–9. 10.3201/eid1202.041115
Silva LA, Vieira RS, Serafini LN, Carlotti Junior CG, Figueiredo JFC: Toxoplasmose do sistema nervoso central em paciente sem evidência de imunossupressão: relato de caso.Rev Soc Bras Med Trop 2001,34(5):487–90.
Kaye A: Toxoplasmosis: diagnosis, treatment, and prevention in congenitally exposed infants.J Pediatr Health Care 2011,25(6):355–64. 10.1016/j.pedhc.2010.04.008
Almeida RAMB, Silva GF, Llanos JC, Winckler CC, Gomes MRB, Biagioni DS, et al.: Cytomegalovirus andToxoplasma gondiiseroprevalence in a Brazilian liver transplant waiting list.J Venom Anim Toxins Incl Trop Dis 2007,13(4):881–4. [http://www.scielo.br/scielo.php?pid=S1678-91992007000400016&script=sci_arttext] 10.1590/S1678-91992007000400016
Fan CK, Su KE, Chung WC, Tsai YJ, Chiou HY, Lin CF, et al.: Seroprevalence ofToxoplasma gondiiantibodies among Atayal aboriginal people and their hunting dogs in northeastern Taiwan.Jpn J Med Sci Biol 1998,51(1):35–42. 10.7883/yoken1952.51.35
Zajdenweber M, Muccioli C, Belfort R Jr: Acometimento ocular em pacientes com AIDS e toxoplasmose do sistema nervoso central - antes e depois do HAART.Arq Bras Oftalmol 2005,68(6):773–5. 10.1590/S0004-27492005000600012
Kung DH, Hubenthal EA, Kwan JY, Shelburne SA, Goodman JC, Kass JS: Toxoplasmosis myelopathy and myopathy in an AIDS patient: a case of immune reconstitution inflammatory syndrome?Neurologist 2011,17(1):49–51. 10.1097/NRL.0b013e3181d35c62
Sabin AB, Feldman HA: Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoon parasite (Toxoplasma).Science 1948,108(2815):660–3. 10.1126/science.108.2815.660
Camargo ME: Introdução às técnicas de imunofluorescência.Rev Bras Patol Clín 1974,10(1):143–71.
Camargo ME: Alguns aspectos atuais do diagnóstico de laboratório da toxoplasmose.An Acad Nac Med 1995, 155:236–9.
Jacobs J, Lunde MN: A hemagglutination test for toxoplasmosis.J Parasitol 1957,43(3):308–14. 10.2307/3274354
Machín Sánchez R, Cruz Castillo F, Prividal Grana J: Comparación de ELISA con las técnicas de inmunofluorescencia indirecta y fijación del complemento para el diagnóstico de la toxoplasmosis.Rev Cuba Med Trop 1985,37(3):269–77.
Ashburn D, Joss AW, Pennington TH, Ho-Yen DO: Specificity and usefulness of an IgE immunosorbent agglutination assay for toxoplasmosis.J Clin Pathol 1995,48(1):64–9. 10.1136/jcp.48.1.64
Furtado JM, Smith JR, Belfort R Jr, Gattey D, Winthrop KL: Toxoplasmosis: a global threat.J Glob Infect Dis 2011,3(3):281–4. 10.4103/0974-777X.83536
Shin DW, Cha DY, Hua QJ, Cha GH, Lee YH: Seroprevalence ofToxoplasma gondiiinfection and characteristics of seropositive patients in general hospitals in Daejeon, Korea.Kor J Parasitol 2009,47(2):125–30. 10.3347/kjp.2009.47.2.125
Xiao Y, Yin J, Jiang N, Xiang M, Hao L, Lu H, et al.: Seroepidemiology of humanToxoplasma gondiiinfection in China.BMC Infect Dis 2010, 10:4. doi:10.1186/1471–2334–10–4 10.1186/1471-2334-10-4
Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB: Toxoplasma gondiiinfection in the United States: seroprevalence and risk factors.Am J Epidemiol 2001,154(4):357–65. 10.1093/aje/154.4.357
Kamani J, Mani AU, Egwu GO, Kumshe HA: Seroprevalence of human infection withToxoplasma gondiiand the associated risk factors, in Maiduguri, Borno state, Nigeria.Ann Trop Med Parasitol 2009,103(4):317–21. 10.1179/136485909X435094
Jaqueti J, Hernández-Garcia R, Nicolás D, Martínez DH, Navarro FG: Serologia frente aToxoplasma gondiien mujere gestantes. Evolución de tasas de prevalência a lo largo de cuatro años.Rev Clin Esp 1991,188(6):278–9.
Swai ES, Schoonman L: Seroprevalence ofToxoplasma gondiiinfection amongst residents of Tanga district in north-east Tanzania.Tanzan J Health Res 2009,11(4):205–9.
Fromont EG, Riche B, Rabilloud M: Toxoplasma seroprevalence in a rural population in France: detection of a household effect.BMC Infect Dis 2009, 9:76. doi:10.1186/1471–2334–9-76 10.1186/1471-2334-9-76
Rodier MH, Berthonneau J, Bourgoin A, Giraudeau G, Agius G, Burucoa C, et al.: Seroprevalences ofToxoplasma, malaria, rubella, cytomegalovirus, HIV and treponemal infections among pregnant women in Cotonou, Republic of Benin.Acta Trop 1995,59(4):271–7. 10.1016/0001-706X(95)00087-U
Secretaria de Vigilância em Saúde: Surto de Toxoplasmose adquirida, Anápolis -GO, fevereiro de 2006. Brasília: Ministério da Saúde; 2007. [http://www.saude.gov.br/svs]
Instituto Brasileiro de Geografia e Estatística (IBGE): Censo Populacional 2010. [http://censo2010.ibge.gov.br/]. Accessed on Octuber 10 2014
Uchôa CMA, Duarte R, Laurentino-Silva V, Alexandre GMC, Ferreira HG, Amendoeira MRR: Padronização de ensaio imunoenzimático para pesquisa de anticorpos das classes IgM e IgG anti-Toxoplasma gondiie comparação com a técnica de imunofluorescência indireta.Rev Soc Bras Med Trop 1999,32(6):661–9. 10.1590/S0037-86821999000600008
Hedman K, Lappalainen M, Seppäiä I, Mäkelä O: Recent primary toxoplasma infection indicated by a low avidity of specific IgG.J Infect Dis 1989,159(4):736–41. 10.1093/infdis/159.4.736
Dean AG: Microcomputers and the future of epidemiology.Public Health Rep 1994,109(3):439–41.
Tanyuksel M, Guney C, Araz E, Saracli MA, Doganci L: Performance of the immunoglobulin G avidity and enzyme immunoassay IgG/IgM screening tests for differentiation of the clinical spectrum of toxoplasmosis.J Microbiol 2004,42(3):211–15.
Reis MM, Tessaro MM, D’Azevedo PA: Toxoplasma-IgM and IgG-avidity in single samples from areas with a high infection rate can determine the risk of mother-to-child transmission.Rev Inst Med Trop Sao Paulo 2006,48(2):93–8. 10.1590/S0036-46652006000200007
Montoya JG, Liesenfeld O: Toxoplasmosis.Lancet 2004,363(9425):1965–76. 10.1016/S0140-6736(04)16412-X
Piergili Fioretti D: Problems and limitations of conventional and innovative methods for the diagnosis of Toxoplasmosis in humans and animals.Parassitologia 2004,46(1–2):177–81. [Article in Italian]
Montoya JG, Liesenfeld O, Kinney S, Press C, Remington JS: VIDAS test for avidity ofToxoplasma-specific immunoglobulin G for confimatory testing of pregnant women.J Clin Microbiol 2002,40(7):2504–8. 10.1128/JCM.40.7.2504-2508.2002
Coura JR (Ed): Dinâmica das doenças infecciosas e parasitárias. Volume II edition. Rio de Janeiro: Guanabara Koogan; 2005.
KPL Technical service Report: Comparison of ABTS, TMB, and OPD peroxidase substrate systems. [http://www.kpl.com/docs/techdocs/ML108.PDF]
Spalding SM, Amendoeira MR, Klein CH, Ribeiro LC: Serological screening and toxoplasmosis exposure factors among pregnant women in South of Brazil.Rev Soc Bras Med Trop 2005,38(2):173–7. 10.1590/S0037-86822005000200009
Navarro IT, Vidotto O, Giraldi N, Freire R: Estudo da resistência doToxoplasma gondiiao efeito do cloreto de sódio e condimentos em linguiça frescal de suínos.OPAS 1992, 112:138–43.
Amendoeira MRR, Costa T, Spalding SM: Toxoplasma gondiiNicolle & Manceaux, 1909 (Apicomplexa: Sarcocystidae) e a Toxoplasmose.Rev Souza Marques 1999,1(1):15–35.
Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP: Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention.Trends Parasitol 2010,26(4):190–6. 10.1016/j.pt.2010.01.009
Souza WJS: Epidemiologia da toxoplasmose: avaliação sorológica de suínos e trabalhadores em abatedouros na mesorregião do Grande Rio de Janeiro. PhD thesis. Universidade Federal Rural do Rio de Janeiro, Toxicology Department; 1995.
Daguer H, Vicente RT, Costa T, Virmond MP, Hamann W, Amendoeira MRR: Soroprevalência de anticorpos anti-Toxoplasma gondiiem bovinos e funcionários de matadouros da microrregião de Pato Branco, Paraná. Brasil.Ciênc Rural 2004,34(4):1133–7.
Sharif M, Daryani A, Barzegar G, Nasrolahei M: A seroepidemiological survey for toxoplasmosis among schoolchildren of Sari, Northern Ira.Trop Biomed 2010,27(2):220–5.
Acknowledgments
The authors would like to thank the Fundação para o Desenvolvimento da Unesp (Fundunesp), the Municipal Government of Assis (SP, Brazil) and CIVAP/Saúde for the financial support. MYM received a grant from the Coordenação para Aperfeiçoamento de Pessoal de Nivel Superior(CAPES) and CAF was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors would also like to thank Roselaine Pereira Alvim Cardoso, Fernanda Evers, Fernando Nakanishi Hamada and Irani Alves for the technical support during the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JTRP, ITN and JPR conceived the project and experimental design. JPR, MS and MYM contributed to the execution and data analysis of the project. FF, ITN, LPS, HFAJ and CAF performed statistical analysis and discussion of the data. All the authors contributed to data analysis, writing, and revision of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rodrigues, J.P., Frei, F., Navarro, I.T. et al. Seroepidemiological analysis of toxoplasmosis in college students. J Venom Anim Toxins Incl Trop Dis 21, 1 (2015). https://doi.org/10.1186/1678-9199-21-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1678-9199-21-1