Abstract
Background
High-mobility group box 1 (HMGB1) is a nucleoprotein that is related to inflammation. It has been implicated in a variety of biologically important processes, including transcription, DNA repair, differentiation, development, and extracellular signaling. Recently, its important role in the process of tumor invasion, metastasis, and resistance to anti-cancer therapies has been demonstrated. In this study, we aimed to investigate the correlation of HMGB1 expression and resistance of rectal cancer patients to chemoradiotherapy (CRT) prior to curative operation.
Methods
We retrospectively reviewed the data of 75 lower rectal cancer patients without complete pathological response who had received preoperative CRT and had undergone curative resection at the University of Tokyo Hospital between May 2003 and June 2010. HMGB1 expression in surgically resected specimens was evaluated using immunohistochemical detection and specimens were classified into high or low HMGB1 expression groups. Clinicopathologic features, degree of tumor reduction, regression of tumor grade, and patient survival were compared between the groups using non-paired Student’s t-tests and Kaplan-Meier analysis.
Results
A total of 52 (69.3%) patients had high HMGB1 expression, and 23 (30.7%) had low expression. HMGB1 expression was significantly correlated with histologic type (P = 0.02), lymphatic invasion (P = 0.02), and venous invasion (P = 0.05). Compared to patients with low HMGB1 expression, those with high expression had a poorer response to CRT, in terms of tumor reduction ratio (42.2 versus 28.9%, respectively; P <0.01) and post-CRT histological tumor regression grade (56.5 versus 30.8% grade 2; respectively; P = 0.03). However, no significant correlation was found between HMGB1 expression and recurrence-free and overall survival rates.
Conclusions
HMGB1 expression may be one of the key factors regulating the response of rectal cancer to preoperative CRT in terms of tumor invasiveness and resistance to therapy.
Similar content being viewed by others
Background
High-mobility group box 1 (HMGB1) was first identified as a nuclear chromatin-binding protein that plays significant roles in various biologically important processes, including transcription, DNA repair, differentiation, and development [1]. In addition to its biological functions in the nuclear compartment, HMGB1 functions as an extracellular signaling molecule during inflammation, cell differentiation, cell migration, and metastasis [2–6]. HMGB1 is reported to be actively secreted by inflammatory cells when stimulated by endotoxin, tumor necrosis factor-α (TNF-α), or interleukin-1β (IL-1β), and is also passively released from necrotic cells [7, 8]. HMGB1 promotes inflammation by binding to the receptors, such as the receptor for advanced glycation end-products (RAGE), Toll-like receptor (TLR) 2, and TLR-4, which are expressed in a variety of cells including monocytes, macrophages, and endothelial cells [7, 9–12]. Through these actions, HMGB1 has been implicated in the pathogenesis of various clinical conditions, including sepsis [13], ischemia-reperfusion [14], meningitis [15], neurodegeneration [16], aging [17], and cancer [5, 6, 18].
In the pathophysiology of cancer, increased expression of HMGB1 and RAGE is associated with proliferative activity and metastatic potential in many types of tumors, including breast cancer [19], hepatocellular carcinoma [8], melanoma [20], glioma [21, 22], prostate cancer [23], gastric cancer [24], and colorectal cancer [25–27]. Increased RAGE-HMGB1 activity induces phosphorylation of extracellular signal-related kinase (ERK) [28], activating GTPases of the Rho family [29]. Thus, it contributes to cancer development through different mechanisms, including angiogenesis [28], cell migration [30], and apoptosis inhibition [24]. Moreover, extracellular reducible HMGB1 has been shown to induce autophagy and promote tumor resistance to alkylators, tubulin disrupting agents, DNA cross-linkers, and DNA intercalators in human pancreatic cancer and colon cancer cell lines [31]. Chemotherapy-induced HMGB1 expression in osteosarcoma cells promotes autophagy to inhibit apoptosis and increase drug resistance [32]. However, HMGB1 has a paradoxical dual effect on tumors [6]. HMGB1 has been shown to stimulate mature dendritic cells to degrade tumor antigen processing through its interaction with TLR-4 [33]. Furthermore, it mediates endogenous TLR-2 activation, resulting in tumor regression [34]. Therefore, the role of HMGB1 in cancer development and progression, as well as its effect on the response to treatment, remains largely unexplored.
Colorectal cancer is a highly invasive and metastatic tumor, and mortality associated with this cancer has increased worldwide recently [35]. Surgical resection and combined modality therapy, including chemotherapy, radiotherapy, and chemoradiotherapy (CRT), are the main therapeutic strategies for the management of rectal cancer. However, the effectiveness of these therapies greatly varies among patients, and those with a weaker response have a worse prognosis. In particular, those who do not respond to neoadjuvant CRT have a poor prognosis [36]. Thus, analysis of the molecular mechanisms underlying the resistance of rectal cancer cells to CRT is essential for the development of novel treatment strategies for the disease. In this study, we performed immunohistochemical analyses to examine HMGB1 expression in surgically resected specimens of rectal cancer after preoperative CRT and investigated its association with clinicopathological features, in an attempt to elucidate the possible association between HMGB1 expression and resistance to CRT.
Methods
Patients and evaluation of response to chemoradiotherapy
A total of 82 patients with lower rectal cancer who had received preoperative CRT and undergone curative resection at the University of Tokyo Hospital between May 2003 and June 2010 were enrolled. Patients receiving CRT had cancer in the middle or lower part of the rectum, with tumor invading further than the muscularis propria. CRT consisted of radiotherapy (1.8 Gy × 28 fractions = 50.4 Gy irradiation) and chemotherapy with a 5-fluorouracil (FU) prodrug (300 mg/m2/day) and leucovorin (75 mg/day), administered orally during the entire course of radiotherapy. We excluded seven patients with complete pathological response after CRT because in this study, we aimed to evaluate the residual cancer cells by immunohistochemical staining. Thus, among the 82 patients, 75 were considered eligible, and HMGB1 expression in surgically resected specimens was evaluated using immunohistochemical analyses. Clinicopathological features were analyzed on the basis of the TNM Classification of Malignant Tumors, Seventh edition, using the International Union Against Cancer (UICC) [37] and World Health Organization (WHO) histological criteria [38]. Post-CRT histological tumor regression was graded according to the seventh edition of the Japanese Guidelines for Clinical and Pathological Studies on Carcinoma of the Colorectum (Table 1) [39]. In this study, both grade 1a and 1b were classified together as grade 1. The reduction ratio was calculated based on the results of barium enema X-ray examination performed before and after CRT. The largest dimension of the tumor, from the same angle, before, and after CRT, was measured, and the post- to pre-CRT ratio was calculated. Patients’ consent and approval of the Ethics Committee of the University of Tokyo were obtained for the use of clinical samples for research purposes.
Immunohistochemical evaluation
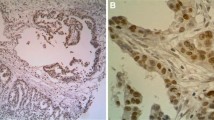
Consecutive formalin-fixed paraffin-embedded 4-μm sections were used for the immunohistochemical evaluation. After treatment with xylene and ethanol, followed by washing with phosphate-buffered saline (PBS), tumor specimens were subjected to heat-induced antigen retrieval in citrate buffer (Muto Pure Chemicals Co., Ltd, Tokyo, Japan). After washing with PBS, endogenous peroxidase was blocked with 3% hydrogen peroxide solution in methanol for 15 minutes (Junsei Chemical Co.Ltd, Tokyo, Japan). The tissues were then washed with PBS and were incubated with 5% bovine serum albumin (BSA) (Sigma Aldrich Chemical Co., St. Louis, Missouri, United States) for 30 minutes to block nonspecific antibody binding. The slides were then incubated overnight at 4°C with monoclonal antibodies against HMGB1 (Sigma Aldrich Chemical Co., St. Louis, Missouri, United States) at a dilution of 1:300. After washing three times with PBS, and incubation with biotinylated rabbit anti-mouse immunoglobulin-labeled globulin (Nichirei, Tokyo, Japan) for 20 minutes, Meyer’s hematoxylin (Sigma Aldrich Chemical Co., St. Louis, Missouri, United States) was used for counterstaining. One field per specimen, from an optimally stained area at a magnification of × 400, was randomly selected for evaluation. HMGB1 expression was strongly and predominantly detected in the nuclei of cancer cells, and it was also weakly observed in the cytoplasm of a few cases (Figure 1). Specimens were classified into high or low HMGB1 expression groups, according to their expression in residual cancer cells. When diffuse HMGB1 staining in the nuclei of residual cancer cells was observed, it was considered as high HMGB1 expression (Figure 1a), and when staining was only observed focally, it was considered as low expression (Figure 1b). Staining was evaluated independently by two observers trained in pathology (KH and SK) who were unaware of the clinical findings. Discrepancies between their findings were resolved by discussion. The correlations between HMGB1 expression and clinicopathological features, tumor recurrence-free survival, and overall survival rates were analyzed.
HMGB1 Immunohistochemical staining of lower rectal carcinoma treated with chemoradiotherapy. (a) High HMGB1 expression (original magnification, ×200). HMGB1 is predominant in the nuclei of tumor cells, and shows diffuse and strong expression. (b) Tumors with low HMGB1 expression (original magnification, ×200). HMGB1 expression is observed in focal tumor cells.
Statistical analysis
The statistical significance of differences was evaluated using non-paired Student’s t-test, as appropriate. An association was considered significant when the exact significance level of the test was less than 0.05. Actuarial overall survival and recurrence-free rates were analyzed using the Kaplan-Meier method. The significance of several variables of the tumor regression grade after CRT was analyzed using logistic regression analysis in multivariate analysis.
Results
Patients’ characteristics
The clinicopathological findings of the 75 patients with lower rectal cancer who had received preoperative CRT and undergone surgical resection are listed in Table 2. A total of 45 patients (60.0%) were male, and 30 (40.0%) were female, with an age range of 33 to 79 years (mean 61.4 ± 10.2 years). There were 23 (30.7%) patients with stage 1 lower rectal cancer, 32 (42.7%) with stage 2, 13 (17.4%) with stage 3, and 7 (9.3%) with stage 4. According to the tumor regression grade, 46 (61.3%) were grade 1 and 29 (38.7%) were grade 2. The average reduction ratio after CRT was 33.0 ± 16.1%.
Correlation between clinicopathological features and HMGB1 expression in rectal cancer patients treated with chemoradiotherapy
The correlation between HMGB1 expression and the clinicopathological characteristics of tumors is shown in Table 3. A total of 52 (69.3%) patients had high HMGB1 expression, and 23 (30.7%) had low expression. No correlation was found between HMGB1 expression and age, gender, tumor size, depth of tumor, lymph node metastasis, and TNM stage. However, HMGB1 expression significantly correlated with the histological type of the tumor (P = 0.02), lymphatic invasion (P = 0.02), and venous invasion (P = 0.05). Well-differentiated tumors were observed in 87% (20 out of 23) of tumors with low HMGB1 expression and 55.8% (29 out of 52) of tumors with high expression (P = 0.02). Lymphatic invasion was identified in 13.5% (seven out of 52) of tumors with high HMGB1 expression, but in no cases with low HMGB1 (P = 0.02). Venous invasion was present in 59.6% (31 out of 52) of tumors with high HMGB1 expression compared to 34.8% (eight out of 23) of those with low expression (P = 0.05). Moreover, compared to low expression, high HMGB1 expression was associated with a poorer response to CRT, in terms of both the tumor reduction ratio (42.2 versus 28.9%, respectively; P <0.01) and the post-CRT histological tumor regression grade (43.5 versus 69.2% and 56.5 versus 30.8%, respectively for grades 1 and 2; P = 0.03).
Recurrence-free survival and overall survival analysis of rectal cancer patients in relation to HMGB1 expression
Using Kaplan-Meier analysis, the log-rank test revealed no significant correlation between the expression of HMGB1 and recurrence-free survival, overall survival (Figure 2a, b), and local recurrence (data not shown).
Discussion
HMGB1 is both a nuclear factor and a secreted protein that acts as a damage-associated molecular pattern molecule (DAMP) [40]. Recently, HMGB1 has been shown to play an important role in cancer biology, including angiogenesis, apoptosis, growth signals, tissue invasion, and metastasis [41–43]. In this study, we focused on the HMGB1 staining pattern in the nuclear compartment, but not the cytoplasm, of rectal cancer cells, and evaluated its possible role in tumor progression and resistance to treatment.
In our series, HMGB1 expression significantly correlated with the histological type of tumor, lymphatic invasion, and venous invasion in rectal cancer. Patients with high HMGB1 expression were more resistant to CRT, as revealed by lower tumor reduction ratio and lower post-CRT histological tumor regression grade. This may imply that rectal cancers with high HMGB1 expression have higher malignancy potential, acquiring resistance to CRT. Corroborating these findings, we observed that HMGB1 expression significantly correlated with lymphatic and venous invasion. In oral cancer, HMGB1 has been reported to promote lymphangiogenesis through the upregulation of vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor D (VEGF-D) [44], which might be linked to the transmigration of HMGB1 [27]. Furthermore, in nasopharyngeal carcinoma cells, endogenous HMGB1 expression was associated with invasiveness [41].
Possible mechanisms of induction of resistance to radiotherapy and chemotherapy by HMGB1 may be: 1) the facilitation of protein-protein interaction and recognition of DNA damage in the process of mismatch repair [45]; 2) regulation of autophagy [31]; and 3) regulation of heat shock protein beta-1 (HSPB1) gene expression, which regulates mitophagy [46]. After DNA damage induced by ultraviolet light irradiation or platination, HMGB1 is sequestered in the nucleus, which is classically associated with apoptosis [8]. An increased level of HMGB1 might promote DNA repair induced by radiation. In addition, HMGB1 is a critical regulator of autophagy [31], promoting drug resistance in osteosarcoma [32] and leukemia cells [47]. Chemotherapy-induced HMGB1 expression in osteosarcoma cells promoted autophagy through controlling the formation of the Beclin 1-phosphatidylinositol 3-kinase class 3 (PI3KC3) complex to inhibit apoptosis and increase drug resistance [32]. Most cancer therapies, such as radiation and anti-cancer drugs, induce cancer cells to undergo autophagy [48, 49], which is an important mechanism of resistance to therapy; thus, mechanisms involving HMGB1 might be key regulators of resistance to anti-cancer therapies. Furthermore, nuclear HMGB1 regulates HSPB1 gene expression. Mitophagy is responsible for the elimination of dysfunctional and impaired mitochondria. It is unclear whether or how mitophagy triggered by dysfunctional mitochondria is regulated by nuclear mediators. However, it has been recognized that HMGB1 modulates mitochondrial respiration and morphological features by helping to sustain autophagy in mitochondrial maintenance through regulation of HSPB1 gene expression [46]. Autophagy and mitophagy, therefore, are involved in sustaining mitochondrial respiration and morphological features after cellular stress and mitochondrial injury. These mechanisms of nuclear HMGB1, including DNA repair, and regulation of autophagy and mitophagy, might be involved in the development of resistance of rectal cancer cells to CRT. Thus, targeting of HMGB1 may be a promising approach for the development of novel therapeutic strategies for rectal cancer.
In our series, however, no significant correlation between HMGB1 expression and recurrence-free or overall survival was found. Since HMGB1 is reported to have paradoxical effects on tumor progression by affecting both the cancer cells and the tumor immunity, the counter-balance between these two effects may be important in determining the final effect. However, caution is required in interpreting our present data. HMGB1 could not be identified as an effective predictive factor for CRT, because only tumor tissues obtained after CRT were used for immunohistochemical staining of HMGB1, and immunostaining of HMGB1 may be affected by CRT itself. Another limitation was the small number of patients, and the retrospective nature of the study.
Conclusions
In conclusion, using immunohistochemistry, this study demonstrated the association of HMGB1 expression in human rectal cancer tissue exposed to CRT with tumor invasiveness and resistance to therapy. HMGB1, which regulates both cell death and cell survival, likely plays a role in the development of carcinogenesis and chemoresistance. Further large-scale prospective studies with long-term follow-up periods, evaluating samples obtained pre- and post-CRT, are needed to determine the potential role of HMGB1 as a prognostic factor for CRT in rectal cancer.
Abbreviations
- CRT:
-
Chemoradiotherapy
- HMGB1:
-
High-mobility group box 1
- HSPB1:
-
Heat shock protein beta-1
- RAGE:
-
Receptors such as advanced glycation end-products
- TLR-2:
-
Toll-like receptor 2
- TLR-4:
-
Toll-like receptor 4
- DAMP:
-
damage associated molecular pattern molecule
- VEGF-C:
-
vascular endothelial growth factor C
- VEGF-D:
-
vascular endothelial growth factor D
- HSPB1:
-
heat shock protein beta-1
- PI3KC3:
-
phosphatidylinositol 3-kinase class 3.
References
Czura CJ, Wang H, Tracey KJ: Dual roles for HMGB1: DNA binding and cytokine. J Endotoxin Res 2001, 7:315–21.
Lotze MT, Tracey KJ: High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005, 5:331–42. 10.1038/nri1594
Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, et al.: New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J 2001, 20:4337–40. 10.1093/emboj/20.16.4337
Dong Xda E, Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, et al.: High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother 2007, 30:596–606. 10.1097/CJI.0b013e31804efc76
Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, et al.: Masquerader: high mobility group box-1 and cancer. Clin Cancer Res 2007, 13:2836–48. 10.1158/1078-0432.CCR-06-1953
Tang D, Kang R, Zeh HJ 3rd, Lotze MT: High-mobility group box 1 and cancer. Biochim Biophys Acta 2010, 1799:131–40. 10.1016/j.bbagrm.2009.11.014
Erlandsson Harris H, Andersson U: Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol 2004, 34:1503–12. 10.1002/eji.200424916
Scaffidi P, Misteli T, Bianchi ME: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418:191–5. 10.1038/nature00858
Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, et al.: Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003, 101:2652–60. 10.1182/blood-2002-05-1300
Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, et al.: Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A 2004, 101:296–301. 10.1073/pnas.2434651100
Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al.: High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 2006, 290:C917–24.
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, et al.: HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 2004, 5:825–30. 10.1038/sj.embor.7400205
Wang H, Yang H, Tracey KJ: Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 2004, 255:320–31. 10.1111/j.1365-2796.2003.01302.x
Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al.: The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. Journal Exp Med 2005, 201:1135–43. 10.1084/jem.20042614
Tang D, Kang R, Cao L, Zhang G, Yu Y, Xiao W, et al.: A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med 2008, 36:291–5. 10.1097/01.CCM.0000295316.86942.CE
Qi ML, Tagawa K, Enokido Y, Yoshimura N, Wada Y, Watase K, et al.: Proteome analysis of soluble nuclear proteins reveals that HMGB1/2 suppress genotoxic stress in polyglutamine diseases. Nat Cell Biol 2007, 9:402–14. 10.1038/ncb1553
Enokido Y, Yoshitake A, Ito H, Okazawa H: Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun 2008, 376:128–33. 10.1016/j.bbrc.2008.08.108
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, et al.: RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 2009, 7:17. 10.1186/1479-5876-7-17
Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, et al.: Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 2004, 164:441–9. 10.1083/jcb.200304135
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al.: HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285:248–51. 10.1126/science.285.5425.248
Yu W, Kim J, Ossowski L: Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy. J Cell Biol 1997, 137:767–77. 10.1083/jcb.137.3.767
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al.: Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405:354–60. 10.1038/35012626
Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H: Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate 2005, 64:92–100. 10.1002/pros.20219
Volp K, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D, et al.: Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut 2006, 55:234–42. 10.1136/gut.2004.062729
Choi YR, Kim H, Kang HJ, Kim NG, Kim JJ, Park KS, et al.: Overexpression of high mobility group box 1 in gastrointestinal stromal tumors with KIT mutation. Cancer Res 2003, 63:2188–93.
Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G: Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol 2010, 136:677–84. 10.1007/s00432-009-0706-1
Moriwaka Y, Luo Y, Ohmori H, Fujii K, Tatsumoto N, Sasahira T, et al.: HMGB1 attenuates anti-metastatic defense of the lymph nodes in colorectal cancer. Pathobiology 2010, 77:17–23. 10.1159/000272950
Yang H, Wang H, Czura CJ, Tracey KJ: The cytokine activity of HMGB1. J Leukoc Biol 2005, 78:1–8. 10.1189/jlb.1104648
Huttunen HJ, Fages C, Rauvala H: Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem 1999, 274:19919–24. 10.1074/jbc.274.28.19919
Bartling B, Hofmann HS, Weigle B, Silber RE, Simm A: Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis 2005, 26:293–301.
Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, et al.: HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 2010, 29:5299–310. 10.1038/onc.2010.261
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, et al.: HMGB1 promotes drug resistance in osteosarcoma. Cancer Res 2012, 72:230–8. 10.1158/0008-5472.CAN-11-2001
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al.: Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007, 13:1050–9. 10.1038/nm1622
Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al.: HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 2009, 6:e10. 10.1371/journal.pmed.1000010
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW: Colorectal cancer. Lancet 2005, 365:153–65. 10.1016/S0140-6736(05)17706-X
Belluco C, De Paoli A, Canzonieri V, Sigon R, Fornasarig M, Buonadonna A, et al.: Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: implications for local excision surgical strategies. Ann Surg Oncol 2011, 18:3686–93. 10.1245/s10434-011-1822-0
Sobin LH, Gospodarowicz MK, Wittekind C: TNM Classification of Malignant Tumors. .7th edition. Hoboken, NJ, United States: Wiley-Blackwell; 2009.
Organnization WH: International Classification of Diseases for Oncology. 3rd edition. International Association of Cancer Registries: Lyon, France; 2000.
Rectum JS, Cot C: General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum, and Anus. Tokyo, Japan: Kanehara & Co; 2006.
Bianchi ME: DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007, 81:1–5.
Mantovani A: Cancer: inflaming metastasis. Nature 2009, 457:36–7.
Mantovani A, Allavena P, Sica A, Balkwill F: Cancer-related inflammation. Nature 2008, 454:436–44. 10.1038/nature07205
Vakkila J, Lotze MT: Inflammation and necrosis promote tumour growth. Nat Rev Immunol 2004, 4:641–8. 10.1038/nri1415
Sasahira T, Kirita T, Oue N, Bhawal UK, Yamamoto K, Fujii K, et al.: High mobility group box-1-inducible melanoma inhibitory activity is associated with nodal metastasis and lymphangiogenesis in oral squamous cell carcinoma. Cancer Sci 2008, 99:1806–12.
Yuan F, Gu L, Guo S, Wang C, Li GM: Evidence for involvement of HMGB1 protein in human DNA mismatch repair. J Biol Chem 2004, 279:20935–40. 10.1074/jbc.M401931200
Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, et al.: High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab 2011, 13:701–11. 10.1016/j.cmet.2011.04.008
Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, et al.: HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia 2011, 25:23–31. 10.1038/leu.2010.225
Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res 2008, 68:1485–94. 10.1158/0008-5472.CAN-07-0562
Levine B: Cell biology: autophagy and cancer. Nature 2007, 446:745–7. 10.1038/446745a
Acknowledgements
We would like to thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests related to this manuscript.
Authors’ contributions
All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hongo, K., Kazama, S., Tsuno, N.H. et al. Immunohistochemical detection of high-mobility group box 1 correlates with resistance of preoperative chemoradiotherapy for lower rectal cancer: a retrospective study. World J Surg Onc 13, 7 (2015). https://doi.org/10.1186/1477-7819-13-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-13-7