Abstract
Background
The effects of fish oil supplements on lipid profile in dialysis patients are controversial. With increasing interest in the potential health benefits of fish oil, it is important to explore its real effects.
Objective
We aimed to identify and quantify the effects of fish oil on triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) in dialysis patients.
Methods
PubMed, EMBASE and the Cochrane Central Register of Controlled Trials were searched for relevant trials of fish oil and lipid profile in dialysis patients. We identified 209 potential studies and included 13 randomized controlled trials. Eligible studies, determined by consensus using predefined criteria, were reviewed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and a meta-analysis was performed.
Results
Compared with the control group, serum TG and TC levels in the fish oil group were reduced by 0.23 mmol/L (95% CI, −0.31, −0.14, P <0.01) and 0.12 mmol/L (95% CI, −0.23, −0.01, P =0.03), respectively. HDL-C levels were increased by 0.20 mmol/L (95% CI, 0.01, 0.40, P <0.01) attributable to fish oil. In contrast, fish oil did not influence serum LDL-C levels. Subgroup analysis showed the effects of fish oil were stronger in subjects with higher baseline TG levels, and the long-term intervention (>12w) demonstrated a tendency towards greater improvement of serum HDL-C and LDL-C levels compared with short-term intervention (≤12 w). However, both of the changes were not statistically significant in meta-regression analysis. There were no obvious difference in effects of different doses and components of fish oil on lipid levels.
Conclusion
Fish oil supplements reduced serum TG and TC levels, and increased HDL-C levels, without affecting LDL-C levels among dialysis patients. It should benefit patients at risk of cardiovascular diseases. Based on randomized controlled trials, we suggested a daily supplement dose of fish oil for dialysis patients of >1 g, but a high dose might not be necessary.
Similar content being viewed by others
Introduction
Dialysis patients have an inordinate risk of cardiovascular disease (CVD), which remains a major cause of morbidity and mortality in this patient group [1, 2]. Dyslipidemia is an established independent risk factor for CVD. It is well known that in patients with end-stage renal disease (ESRD), changes in lipid metabolism occur, creating a complex form of dyslipidemia [3], and the lipid abnormalities persist or are aggravated during renal replacement treatment [4, 5], which may partly explain the high incidence of CVD [6].
In the past few years, there has been a growing scientific and public interest in the role of omega-3 fatty acids, mainly obtained from fish and fish oil in CVD, idiopathic IgA nephropathy, lupus nephritis and renal failure. By modulating cell membrane structure and function as well as synthesis of lipid mediators such as eicosanoids, omega-3 fatty acid supplementation may offer multiple health benefits to dialysis patients [7].
Numerous clinical trials concerning the effects of fish oil supplements on serum lipids in dialysis patients have been published. However, the results are still inconclusive, because the trials included small numbers of patients and different doses of fish oil, with short duration of observation. Therefore, we present the results of a systematic review summarizing current evidence from randomized controlled trials of the effects of fish oil supplements on serum lipid profile in dialysis patients.
Methods
Search strategy
The systematic review was conducted in accordance with PRSIMA guidelines [8]. The search used key words related to dialysis (kidney failure, chronic renal failure, dialysis, hemodialysis, peritoneal dialysis), omega-3 fatty acids (fatty acid, omega-3, fish oil, α-linolenic acid, eicosapentaenoic acid, docosahexanoic acid) and lipid (lipid, cholesterol, triglyceride, lipoprotein, hyperlipidemia) to identify randomized controlled trials published in the English language covering the period from as early as possible to October 2013, from different computerized databases including PubMed, EMBASE and the Cochrane Central Register of Controlled Trials. In addition, the reference lists of the published papers on clinical trials, review articles and meta-analysis were hand-searched for other relevant studies. The investigators were contacted for unreported lipid data in published trials. The titles and abstracts of the articles were analyzed to ascertain conformity with the inclusion criteria. The full text of an article was reviewed carefully if the screening of its title and abstract was unclear as to its admissibility. The complete search strategy is available in the Additional file 1.
Selection criteria
We included published completed studies that enrolled dialysis patients, regardless of the type of dialysis. Randomized controlled trials were included if they met the following criteria: (i) the study compared fish oil (any dose or type) versus placebo/no treatment; (ii) concomitant therapy with fish oil equally in both treatment arms; and (iii) at least one of the following four outcomes were reported: serum TG, TC, LDL-C, HDL-C. All the studies were reviewed by two independent reviewers (Han Du, He Zhang) and any disagreement was resolved by discussion.
Quality assessment
To comply with the Cochrane Handbook for Systematic Reviews of Interventions in terms of quality assessment of the randomized controlled trials, we evaluated the quality of the studies in terms of allocation concealment and intention-to-treat analysis, blinding of investigators, participants and outcome assessors, and completeness to follow-up, as well as the Jadad scale [9–11]. The Jadad scale was performed by two reviewers independently (Wei Zhu, Jie Chen).
Data extraction
We followed a standard Cochrane protocol for study selection and data extraction [12, 13]. Study eligibility was determined by consensus, based on previously determined inclusion criteria. Eligible studies were reviewed independently by two authors (Wei Zhu, Feng Hu) who used data extraction forms developed for this purpose. We used consensus and a third reviewer (Chongya Dong), if necessary, to resolve disagreements. Data were extracted from all included trials in terms of baseline characteristics of the trials, type of dialysis, the components and dose of fish oil, follow-up duration, and the following reported outcomes: serum TG, TC, LDL-C, and HDL-C. If an outcome was reported at more than one time point in a single study, the longest period of follow-up was used.
Data synthesis and statistical analysis
The mean difference in TG, TC, HDL-C and LDL-C from baseline was extracted into meta-analysis as a continuous variable. The effects of fish oil compared with control oil on the lipid profile were defined as the mean difference between the changes in serum lipid concentration. The mean changes were calculated by subtracting the baseline values from the final values. For studies only reporting data of the pre-intervention mean and SD and the post-intervention mean and SD, we inputted the missing SD of changes from baseline using a correlation coefficient of 0.7, as calculated and averaged based on studies with complete outcome reports [14–17]. For cross-over studies, the correlation coefficient was 0.797, as calculated based on a complete outcome report with cross-over design [18].
Heterogeneity among studies was assessed using Cochran’s Q test and I2 statistic. P <0.05 or I2 > 50% was considered significant. In that case, the random effects model was used as the pooling method. Otherwise, the fixed effect model was chosen. Funnel plots and Egger’s regression were used to assess the potential publication bias.
We excluded the study by Taziki after the data synthesis phase [19], and the correlation coefficient was not available in primary reports, therefore we performed a sensitivity analysis.
The components and dose of fish oil, duration of intervention, and initial lipid levels may influence the results, therefore we performed subgroup comparisons based on the parameters above. Because these previous studies measured fish oil as a whole and it has been reported that DHA and EPA content varied in different types of fish oil, we also subgrouped the trials on the basis of the dose of DHA + EPA in the included trials. Meta-regression was performed to compare the effects of these subgroup characteristics on the outcomes.
Review Manager 5.2 created by the Cochrane Collaboration for meta-analysis (http://www.cochrane.org) was used for statistical analysis.
Role of the funding source
This study had no funding source. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Literature search
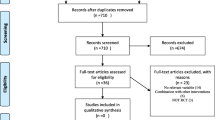
A total of 209 articles were found in our initial search, and 178 were excluded by screening the titles or abstracts, because they were animal studies, basic research studies, review articles, or non-randomized controlled trials, and they did not enroll dialysis patients or compare fish oil versus placebo/no treatment. Full-text assessment of the 31 potentially relevant articles resulted in 13 eligible randomized controlled studies. The reasons for exclusion were as follows: one article was a review one trial was not randomized and controlled trial, one full-text was a study protocol, and 13 studies did not report sufficient lipid detail for inclusion in the meta-analysis. Two studies had the same first author, dialysis clinics, start and end point, inclusion and exclusion criteria [20, 21], and we confirmed that 33 patients had been included in both studies. In the absence of prompt availability of patient-level data for both trials, we only included the trial with the highest number of patients [20]. One trial was excluded because we found the data quality questionable. Unfortunately, we were unable to contact the author to confirm the data quality. Finally, all the authors agreed to exclude it after discussion. The reasons were as follows: (i) Fasting serum TC should be >220 mg/dl as per the inclusion criteria. However, the baseline levels provided for both groups (102 ± 32 and 229 ± 31 mg/dl) were not within the inclusion criteria [22]; (ii) The baseline TG level in the drug group was significantly higher than in the control group (321 ± 29 vs 268 ± 22 mg/dl), and the baseline TC level was significantly lower (102 ± 32 vs 229 ± 31 mg/dl). Both groups should have matching data. This could be the cause of the apparent benefit with fatty acid supplementation. The data regarding baseline TG of the control group in the two tables are different (268 ± 22 and 268 ± 32 mg/dl) [22]. We included the study by Poullia in spite of patients received different α-tocopherol doses as concomitant therapy [18], because the supplement aimed to control the possible effects of α-tocopherol present in Omacor capsules, and had no impact on lipid profile [23]. A flow diagram of the article selection for this meta-analysis is shown in Figure 1.
Quality assessment
Quality assessment of the primary studies is summarized in Table 1. The Jadad scores ranged from 0 to 5 points. Study quality on the whole was good; five of 13 studies had a Jadad score of 5, four of 13 had a Jadad score of 3 or 4, and four of 13 had a Jadad score of ≤2. Participants and investigators were blinded in nine of 13 trials; sufficient details of drop-outs and withdrawals were described in 13 trials, two studies complied with allocation concealment; and 4 studies met the intention-to-treat analysis criteria.
Study characteristics
We identified 13 trials with 764 subjects in our study [14–18, 20, 24–30]. The characteristics of the trials are shown in Table 2. One study focused on hyperlipidemia patients. One study enrolled subjects with serum albumin ≤3.9 g/dl. Eleven of the trials were parallel trials and two were cross-over studies. Ten of the trials enrolled patients on hemodialysis, one trial on peritoneal dialysis, and two on both peritoneal dialysis and hemodialysis. Duration of fish oil supplementation was 4 weeks to 12 months. The daily amount of EPA + DHA supplement ranged from 0.9 g (dose of EPA + DHA was estimated as 60% of fish oil dose) to 3 g [16, 19]. All 13 studies reported the effects on TG levels; 12 studies reported the effects on cholesterol, and nine and eight studies investigated the effects of fish oil on HDL-C and LDL-C, respectively.
Effects of fish oil supplementation on lipid concentrations
The effects of fish oil on the levels of TG, TC, HDL-C and LDL-C in all studies are shown in Figures 2, 3, 4 and 5, respectively. Overall, the pooled analysis of the effects of fish oil intake on TG levels showed a decrease of 0.23 mmol/L compared with the control group (95% CI, −0.31, −0.14, P <0.01) (Figure 2). Fish oil also significantly lowered serum TC levels by 0.12 mmol/L (95% CI, −0.23, −0.01, P =0.03) (Figure 3). Fish oil significantly increased HDL-C levels by 0.20 mmol/L (95% CI, 0.01, 0.40, P <0.01). Heterogeneity was observed for the HDL-C outcome (heterogeneity chi-square = 836.86, I2 = 97%, P = 0.04) (Figure 4). Fish oil did not have any significant influence on LDL-C (mean difference −0.03 mmol/L; 95% CI, −0.15, 0.09, P = 0.62) (Figure 5).
Publication bias
The potential publication bias was detected by funnel plots and Egger’s regression test (Figure 6). The results suggested no publication bias for the effects of fish oil on the parameters, including TC, TG and LDL-C. However, funnel plots revealed that publication bias existed for HDL-C, which was also illustrated by Egger’s regression test (P <0.01). It may have been caused by two articles whose results deviated from the others [20, 27], and negative results about HDL-C are published less often.
Subgroup analyses
The results of the subgroup analyses are shown in Table 3. The effects of fish oil on serum TG were found to be greater in patients with higher baseline TG levels. The mean change in TG in the subgroups with baseline levels ≥2.26, 1.69-2.26 and ≤1.69 mmol/L was −0.56, −0.18 and −0.24 mmol/L, respectively. However, no significance could be found in meta-regression analysis (P =0.75).
Long-term (>12 w) intervention demonstrated a tendency towards greater improvement in serum HDL-C and LDL-C levels compared with short-term intervention (≤12 w). The mean change in HDL-C in the ≤12 w and >12 w subgroups was 0.12 and 0.44 mmol/L, respectively, and the change in LDL-C in ≤12 w and >12 w subgroups was 0.06 and −0.12 mmol/L, respectively. However, meta-regression analysis showed no significant association between serum HDL-C or LDL-C outcomes and duration of intervention (P =0.12, P =0.31). There was no significant difference in the effects between doses and components of fish oil on lipid levels.
Sensitivity analysis
For sensitivity analysis, because we used a correlation coefficient of 0.7 to input the missing SD of changes from baseline as calculated and averaged based on studies with complete outcome reports, we also inputted correlation coefficients of 0.5 and 0.9 [31]. This had no effect on the significance of the pooled changes in TG, TC, LDL-C and HDL-C.
Although the trial by Taziki was excluded [19], we also analyzed the results that included Taziki’s trial data. We compared the results when we included and excluded Taziki’s trial data. The significance of the pooled changes in TG, TC, LDL-C and HDL-C was not altered, except that including the Taziki data changed the homogeneity of the TG and TC studies (I2: TG 29 to 73%, TC 27 to 72%).
The results of two studies deviated from others obviously, which may be the reason for the positive publication bias result for HDL-C [20, 27]. Therefore, we analyzed the results after excluding these data, but reached the same conclusion as before. Fish oil did not have any significant influence on HDL-C (mean difference −0.01 mmol/L; 95% CI, −0.03, 0.02 mmol/L, P =0.90).
Discussion
Our meta-analysis showed supplementation with fish oil was associated with a decrease in TG and TC, and an increase in HDL-C, but had no significant effects on LDL-C compared with controls. Funnel plots and Egger’s regression test showed possible publication bias for HDL-C, but not for other parameters.
The results of the current meta-analysis on TC, TG, LDL-C and HDL-C were similar to those of Pei [32], in which fish oil was found to reduce serum TG levels significantly by −0.78 mmol/L (95% CI: −1.12, −0.44 mmol/L, P <0.01), but had no significant effect on TC, HDL-C and LDL-C compared with controls. However, there were several differences between the two studies. The two studies by Svensson and Bouzidi [33, 34] were included in the study of Pei, but were excluded in the current study, because the patients enrolled in the two studies did not undergo dialysis treatment. We were able to confirm that two studies of Bowden were based on the same population [20, 21], so we excluded the smaller sample study [21]. The trial by Taziki was excluded in our meta-analysis [19], but included in the analysis of Pei. We also added seven studies not included in Pei’s review because of small sample sizes (<15 patients in either arm) and search dates [15–18, 24, 29, 30]. Furthermore, we added several subgroup analyses based on components of fish oil and initial lipid levels.
Subgroup analyses revealed good long-term trends in the effects of supplements of fish oil on serum HDL-C and LDL-C levels. However, as mentioned before, the degree of improvement did not achieve statistical significance, which may have been due to a lack of statistical power. If we want to clarify the long-term effect, more specific studies should be conducted.
There are two issues that need to be carefully scrutinized. The first is that the optimal daily dose in dialysis patients that has a lipid-altering effect is still not established. In our study, the content of fish oil supplements varied significantly. It is impossible to determine the precise content of each supplement, thus the dose of DHA and EPA varied, so we used the dose of EPA + DHA for subgroup analysis. We found no difference in the effects on lipid profile when comparing <2 g EPA + DHA supplement with ≥2 g, therefore a high dose of fish oil supplement for lowering lipid levels might not be needed. As a result of dietary restrictions on fish consumption, to control phosphorus intake, dialysis patients have significantly lower levels of omega-3 fatty acids in their erythrocyte membranes [35], and based on randomized controlled trials, we suggest that the daily supplement dose of fish oil for dialysis patients should be >1 g. This is different from the current recommended dose of up to 1 g/day, by the American Heart Association (AHA) and various international health organizations [36, 37].
Second, the truly active component of fish oil accounting for the lipid-altering effects is still unclear. After a period of DHA supplementation, Harrison reported no significant changes in LDL-C or TC, and a concomitant increase in HDL-C. The authors further speculated that only EPA has lipid-modulating effects [38]. We tried to establish whether EPA and DHA had a different impact on lipid profile through subgroup analyses. Our study confirmed that EPA had a significant influence on lipid profile, and EPA + DHA showed the same lipid-modulating effects as EPA alone. Unfortunately, we could not draw any conclusion about the effect of DHA alone on lipid profile in dialysis patients.
The lipid-altering effects of fish oil may be attributed to many factors [7]. Cell membrane fatty acids play an important role in signal transduction, therefore omega-3 fatty acids are capable of modifying gene expression. It is believed that the dramatic lipid-altering effects of omega-3 fatty acids are mediated via this mechanism [39]. Specifically, omega-3 fatty acids modulate the function of peroxisome proliferator-activated receptors and sterol regulatory binding proteins, both of which are involved in lipid homeostasis [40, 41].
Although fish oil is generally well tolerated, attention should be paid to the safety of fish oil supplementation at doses >1 g/day. Most adverse effects involve a transient minimal decrease in the desire for food [27], gastrointestinal complaints (e.g. gas, bloating, fishy aftertaste [28], nausea and burping [14]), and prolonged bleeding time [25]. Serious bleeding complications were restricted to a single patient in one study [24]. However, the majority of studies were not designed to measure side effects and tolerability after long-term use.
CVDs are the most important cause of mortality in patients undergoing hemodialysis. The frequency of CVD in dialysis patients has been reported as 3–45 times that observed in the general population and 50% of deaths in these patients are related to CVD [42]. Lipid abnormalities often complicate chronic renal failure and persist or are aggravated during renal replacement treatment [4, 43]. Large cohort studies have shown an inverse association between cardiovascular morbidity and mortality and fish oil ingestion [44, 36]. Lok found that among patients with new hemodialysis grafts, daily ingestion of fish oil improved cardiovascular event-free survival [16]. Friedman showed that long-chain omega-3 fatty acids are strongly and independently associated with a lower risk of sudden cardiac death in hemodialysis patients throughout the first year of dialysis [45]. Except for lipid modulation, fish oil may reduce cardiovascular events by several mechanisms, including anti-inflammatory, antiarrhythmic and plaque-stabilizing effects as well as improved endothelial effects [46, 47]. However the potential benefits of fish oil on cardiovascular events deserve confirmation in future studies.
There were some strengths and limitations to our study. We had more strict inclusion criteria, and analyzed almost all the lipid parameters mentioned in the literature. A series of important subgroup analyses were also performed. However, the following limitations must be mentioned. First, none of the trials was sufficiently powered because of the small numbers of participants. Second, until now, there has been insufficient evidence for the effectiveness of long-term supplementation with fish oil on lipid profile, because the longest duration in these trials was only 48 weeks [12]. Third, publication bias is a major potential limitation of meta-analysis that has been minimized by contacting investigators for unpublished results. Funnel plots and Egger’s regression test showed possible publication bias for HDL-C, and sensitivity analysis found that the significance of the change in HDL-C level was altered. We must interpret the HDL result with caution. Finally, none of the studies were specifically designed to assess the effects of fish oil in dyslipidemia patients, thus it was not accurate to evaluate the lipid-altering effects in this group. Based on the above, the results derived from this meta-analysis should be treated with considerable caution.
Conclusions
This meta-analysis suggests that fish oil reduces serum TG and TC levels, and increases HDL-C levels, without influencing LDL-C levels among patients undergoing dialysis treatment. This effect may directly account for part of the observed benefits of fish intake on cardiovascular risk. Based on randomized controlled trials, we suggest that the daily supplement dose of fish oil for dialysis patients should be >1 g, but a high dose might not be necessary. Future large-scale randomized trials with adequate doses and duration of treatment are needed to determine the effect of fish oil supplements on lipid profile in dialysis patients, especially those with CVD.
References
Levin A, Foley RN: Cardiovascular disease in chronic renal insufficiency. Am J Kidney Dis. 2000, 36 (6 Suppl 3): S24-S30.
Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT: Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? what do we need to learn? where do we go from here? national kidney foundation task force on cardiovascular disease. Am J Kidney Dis. 1998, 32 (5): 853-906.
Kwan BC, Kronenberg F, Beddhu S, Cheung AK: Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007, 18 (4): 1246-1261.
Ma KW, Greene EL, Raij L: Cardiovascular risk factors in chronic renal failure and hemodialysis populations. Am J Kidney Dis. 1992, 19 (6): 505-513.
Appel GB: Lipid abnormalities in renal disease. Kidney Int. 1991, 39 (1): 169-183.
Uhlig K, Levey AS, Sarnak MJ: Traditional cardiac risk factors in individuals with chronic kidney disease. Semin Dial. 2003, 16 (2): 118-127.
Friedman A, Moe S: Review of the effects of omega-3 supplementation in dialysis patients. Clin J Am Soc Nephrol. 2006, 1 (2): 182-192.
Moher D, Liberati A, Tetzlaff J, Altman DG, : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009, 151 (4): 264-269.
Jüni P, Altman DG, Egger M: Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001, 323 (7303): 42-46.
Schulz KF, Chalmers I, Hayes RJ, Altman DG: Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995, 273 (5): 408-412.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clinical Trials. 1996, 17 (1): 1-12.
Furlan AD, Pennick V, Bombardier C, van Tulder M, Board E, Group CBR: updated method guidelines for systematic reviews in the Cochrane back review group. Spine (Phila Pa 1976) 2009. 2009, 34 (18): 1929-1941.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000, 283 (15): 2008-2012.
Saifullah A, Watkins BA, Saha C, Li Y, Moe SM, Friedman AN: Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients-a pilot study. Nephrol Dial Transplant. 2007, 22 (12): 3561-3567.
Kooshki A, Taleban FA, Tabibi H, Hedayati M: Effects of omega-3 fatty acids on serum lipids, lipoprotein (a), and hematologic factors in hemodialysis patients. Ren Fail. 2011, 33 (9): 892-898.
Lok CE, Moist L, Hemmelgarn BR, Tonelli M, Vazquez MA, Dorval M, Oliver M, Donnelly S, Allon M, Stanley K: Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: a randomized controlled trial. JAMA. 2012, 307 (17): 1809-1816.
Daud ZA, Tubie B, Adams J, Quainton T, Osia R, Tubie S, Kaur D, Khosla P, Sheyman M: Effects of protein and omega-3 supplementation, provided during regular dialysis sessions, on nutritional and inflammatory indices in hemodialysis patients. Vasc Health Risk Manag. 2012, 8: 187-195.
Poulia KA, Panagiotakos DB, Tourlede E, Rezou A, Stamatiadis D, Boletis J, Zampelas A: Omega-3 fatty acids supplementation does not affect serum lipids in chronic hemodialysis patients. J Ren Nutr. 2011, 21 (6): 479-484.
Taziki O, Lessan-Pezeshki M, Akha O, Vasheghani F: The effect of low dose omega-3 on plasma lipids in hemodialysis patients. Saudi J Kidney Dis Transplant. 2007, 18 (4): 571-576.
Bowden RG, Jitomir J, Wilson RL, Gentile M: Effects of omega-3 fatty acid supplementation on lipid levels in endstage renal disease patients. J Ren Nutr. 2009, 19 (4): 259-266.
Bowden RG, Wilson RL, Deike E, Gentile M: Fish oil supplementation lowers C-reactive protein levels independent of triglyceride reduction in patients with end-stage renal disease. Nutr Clin Pract. 2009, 24 (4): 508-512.
Hari Kumar KV, Verma A, Shekhar , Modi KD: Controversial role of omega-3 fatty acids in dyslipidemia. Saudi J Kidney Dis Transpl. 2008, 19 (2): 254-255.
Shekelle PG, Morton SC, Jungvig LK, Udani J, Spar M, Tu W, J Suttorp M, Coulter I, Newberry SJ, Hardy M: Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med. 2004, 19 (4): 380-389.
Diskin CJ, Thomas CE, Zellner CP, Lock S, Tanja J: Fish oil to prevent intimal hyperplasia and access thrombosis. Nephron. 1990, 55 (4): 445-447.
Donnelly SM, Ali MA, Churchill DN: Effect of n-3 fatty acids from fish oil on hemostasis, blood pressure, and lipid profile of dialysis patients. J Am Soc Nephrol. 1992, 2 (11): 1634-1639.
Ando M, Sanaka T, Nihei H: Eicosapentanoic acid reduces plasma levels of remnant lipoproteins and prevents in vivo peroxidation of LDL in dialysis patients. J Am Soc Nephrol. 1999, 10 (10): 2177-2184.
Khajehdehi P: Lipid-lowering effect of polyunsaturated fatty acids in hemodialysis patients. J Ren Nutr. 2000, 10 (4): 191-195.
Schmitz PG, Mccloud LK, Reikes ST, Leonard CL, Gellens ME: Prophylaxis of hemodialysis graft thrombosis with fish oil: double-blind, randomized, prospective trial. J Am Soc Nephrol. 2002, 13 (1): 184-190.
Svensson M, Schmidt EB, Jørgensen KA, Christensen JH: The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: a randomized placebo-controlled intervention study. Nephrol Dial Transplant. 2008, 23 (9): 2918-2924.
An WS, Lee SM, Son YK, Kim SE, Kim KH, Han JY, Bae HR, Park Y: Effect of omega-3 fatty acids on the modification of erythrocyte membrane fatty acid content including oleic acid in peritoneal dialysis patients. Prostaglandins Leukot Essent Fatty Acids. 2012, 86 (1–2): 29-34.
Follmann D, Elliott P, Suh I, Cutler J: Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992, 45 (7): 769-773.
Pei J, Zhao YY, Huang LJ, Zhang XY, Wu Y: The effect of n-3 polyunsaturated fatty acids on plasma lipids and lipoproteins in patients with chronic renal failure–a meta-analysis of randomized controlled trials. J Ren Nutr. 2012, 22 (6): 525-532.
Svensson M, Christensen JH, Sølling J: The effect of n-3 fatty acids on plasma lipids and lipoproteins and blood pressure in patients with CRF. Am J Kidney Dis. 2004, 44 (1): 77-83.
Bouzidi N, Mekki K, Boukaddoum A, Dida N, Kaddous A, Bouchenak M: Effects of omega-3 polyunsaturated fatty-acid supplementation on redox status in chronic renal failure patients with dyslipidemia. J Ren Nutr. 2010, 20: 321-328.
Koorts AM, Viljoen M, Kruger MC: Red blood cell fatty acid profile of chronic renal failure patients receiving maintenance haemodialysis treatment. Prostaglandins Leukot Essent Fatty Acids. 2002, 67 (1): 13-18.
Kris-Etherton PM, Harris WS, Appel L, : Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002, 106 (21): 2747-2757.
Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM: n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006, 83 (6 Suppl): 1526S-1535S.
Harrison RA, Sagara M, Rajpura A, Armitage L, Birt N, Birt CA, Yamori Y: Can foods with added soya-protein or fish oil reduce risk factors for coronary artery disease? A factorial randomized controlled trial. Nutr Metab Cardiovasc Dis. 2004, 14 (6): 344-350.
Lapillonne A, Clarke SD, Heird WC: Polyunsaturated fatty acids and gene expression. Curr Opin Clin Nutr Metab Care. 2004, 7 (2): 151-156.
Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM: Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997, 94 (9): 4318-4323.
Xu J, Nakamura MT, Cho HP, Clarke SD: Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem. 1999, 274 (33): 23577-23583.
Jungers P, Nguyen Khoa T, Massy ZA, Zingraff J, Labrunie M, Descamps-Latscha B, Man NK: Incidence of atherosclerotic arterial occlusive accidents in predialysis and dialysis patients: a multicentric study in the Ile de France district. Nephrol Dial Transplant. 1999, 14 (4): 898-902.
Majumdar A, Wheeler DC: Lipid abnormalities in renal disease. J R Soc Med. 2000, 93 (4): 178-182.
De Caterina R: n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011, 364 (25): 2439-2450.
Friedman AN, Yu Z, Tabbey R, Denski C, Tamez H, Wenger J, Thadhani R, Li Y, Watkins BA: Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 2013, 83 (6): 1130-1135.
Adkins Y, Kelley DS: Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010, 21 (9): 781-792.
Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF: Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003, 361 (9356): 477-485.
Acknowledgments
We thank Charmaine E. Lok for sharing unpublished results for this analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WZ and CD contributed to the design of the study, data analysis and manuscript. FH, HZ and HD performed articles review. JC and XH helped with the data analysis. All authors read and approved the final manuscript.
Wei Zhu, Chongya Dong contributed equally to this work.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zhu, W., Dong, C., Du, H. et al. Effects of fish oil on serum lipid profile in dialysis patients: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis 13, 127 (2014). https://doi.org/10.1186/1476-511X-13-127
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-511X-13-127