Abstract
Background
Levels of haemoglobin A1c (HbA1c) and blood lipids are important determinants of risk in patients with diabetes. Standard analysis methods based upon venous blood samples can be logistically challenging in resource-poor settings where much of the diabetes epidemic is occurring. Dried blood spots (DBS) provide a simple alternative method for sample collection but the comparability of data from analyses based on DBS is not well established.
Methods
We conducted a systematic review and meta-analysis to define the association of findings for HbA1c and blood lipids for analyses based upon standard methods compared to DBS. The Cochrane, Embase and Medline databases were searched for relevant reports and summary regression lines were estimated.
Results
705 abstracts were found by the initial electronic search with 6 further reports identified by manual review of the full papers. 16 studies provided data for one or more outcomes of interest. There was a close agreement between the results for HbA1c assays based on venous and DBS samples (DBS = 0.9858venous + 0.3809), except for assays based upon affinity chromatography. Significant adjustment was required for assays of total cholesterol (DBS = 0.6807venous + 1.151) but results for triglycerides (DBS = 0.9557venous + 0.1427) were directly comparable.
Conclusions
For HbA1c and selected blood lipids, assays based on DBS samples are clearly associated with assays based on standard venous samples. There are, however, significant uncertainties about the nature of these associations and there is a need for standardisation of the sample collection, transportation, storage and analysis methods before the technique can be considered mainstream. This should be a research priority because better elucidation of metabolic risks in resource poor settings, where venous sampling is infeasible, will be key to addressing the global epidemic of cardiovascular diseases.
Similar content being viewed by others
Background
Cardiovascular diseases [1] are increasing particularly rapidly in developing country settings with diabetes a key determinant of risk [2]. Documenting the role of dysglycaemia and other metabolic risk factors [3–8] can be challenging in these countries because the infrastructure and resources required to conduct research are limited. For example, assays of glycosylated haemoglobin (HbA1c) and blood lipids are usually done on venous blood samples which can be difficult to collect, transport and store. The use of dried blood spot sampling (DBS) [9, 10] is one possible solution. DBS involves pricking the participant’s finger with a lancet and collecting drops of blood on a piece of filter paper. Samples are then dried and placed in sealed plastic bags for transportation and storage [11, 12]. Compared to venous samples, collecting DBS requires minimal training of staff, is cheaper, is safer, provides for simpler transportation and is more acceptable to study participants [12–15].
DBS samples are now widely used for measuring serum antibodies, human immunodeficiency virus (HIV) loads and blood hormone levels with good data to define the comparability of results between analyses based upon DBS and standard venous samples [11, 14, 16, 17]. The absence of comparable data to define the associations for HbA1c and blood lipids means that DBS samples are not widely used in studies making assessment of cardiovascular risks. The objective of this project was to synthesise the available evidence describing the comparability of findings for assays of HbA1c and blood lipids based upon DBS samples compared to standard venous samples.
Methods
This project was a systematic review and meta-analysis done to define the association of findings for HbA1c and blood lipids for analyses based upon standard venous samples compared to DBS samples. This was a secondary analysis of existing published data and no ethics review was therefore required.
Search strategy
The Cochrane, Embase and Medline databases were searched electronically during July 2012 using combinations of the terms “dried blood spot”, “dried blood”, “DBS”, “filter paper”, “triglycerides”, “triacylglycerides”, “HbA1c”, “glycosylated haemoglobin”, “glycosylated hemoglobin, “glycated haemoglobin”, “glycated hemoglobin, “cholesterol”, “high density lipoprotein”, “HDL”, “low density lipoprotein” or “LDL”. Additional studies were identified by a manual examination of the reference lists of all studies identified as eligible.
Eligibility criteria
Studies were eligible for inclusion if they directly compared values generated from analyses based on DBS samples to analyses based on venous samples. To be included, a study had to report in the form of a regression equation an association for one or more of the specified outcomes. There was no restriction on the type of study population.
Data extraction
Two independent observers (EA and PD) reviewed the abstracts for eligibility and extracted standardised data into a data collection sheet for eligible studies. The data sought from each study were based upon a comparable prior systematic review done in the HIV field [17] and included: date of publication, study size, participant characteristics and sample storage conditions. For each risk factor reported upon we sought to identify the laboratory extraction method, biochemical assay method and regression coefficient. Where available we also noted data describing the stability of the DBS samples.
Outcomes
The outcomes studied in this overview were HbA1c, total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol and triglycerides.
Statistical analysis
The characteristics of included studies were summarised in tabular form (Table 1). The linear regression coefficients from each study were pooled separately for each risk factor using a weighted least squares approach [18] to estimate an overall coefficient. The same method was used to estimate a combined intercept. This gave a relationship of the form: DBS = b Venous + a where ‘b’ is the combined coefficient and ‘a’ is the combined intercept. The synthesis of the parameters was done as follows:
where the weight, w i = the number of participants in study i, and bi and ai represent the coefficient and intercept, respectively, for the regression line in study i.
Heterogeneity of the individual study estimates contributing to each meta-analysis was assessed using the Cochran’s Q and I2 statistics. Subsidiary analyses were done to explore the impact of assay method for the outcome of HbA1c.
Results
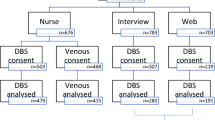
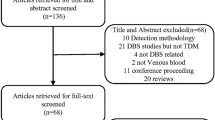
There were 705 records identified by the electronic search for which abstracts were reviewed. Six further studies were found by the manual search of reference lists for included studies (Figure 1). One final study was found during the review process. Sixteen studies were ultimately included in the meta-analysis, 12 of which reported necessary data for HbA1c, 1 for triglycerides, 2 for both triglycerides and total cholesterol and 1 for HbA1c, total cholesterol and HDL (Tables 1, 2) [9, 10, 19, 21–34]. One other study of HbA1c was excluded because it did not provide a regression equation [35] and one other study of triglycerides was excluded because it did not provide the sample size [34]. There were no studies reporting data for LDL-cholesterol identified.
Flow chart detailing identification of studies[36].
The total numbers of participants providing data were 1425 for HbA1c, 773 for triglycerides and 1093 for total cholesterol. Study sizes ranged from 30 to 613 participants. The assay methods varied for HbA1c which included immunoturbidimetric, high performance liquid chromatography (HPLC) and affinity chromatography assays but all studies measuring triglycerides, total cholesterol and HDL used colorimetry.
HbA1c
For HbA1c the summary regression (DBS = 0.9858 V + 0.3809) (Figure 2) showed close agreement between analyses based upon the venous and DBS sampling methods. There was, however, evidence of heterogeneity between the contributing regression lines for the intercepts (Cochran’s Q-test p < 0.001 and I2 = 98%) but not the slopes (p = 0.833 and I2 = 0%). Subsidiary analyses by assay method showed that the heterogeneity was partially attributable to different results for the two studies that used affinity chromatography. Funnel plots did not provide clear evidence of publication bias (Additional file 1: Figures S1 and S2).
Individual (solid) and summary (dotted) regression lines showing the associations between results for analyses based upon dried blood spot (DBS) compared to venous (V) samples for haemoglobin A1 c (HbA1 c ) analysed by any method (2A), HbA1 c analysed by specific methods (2B to 2D), triglycerides (2E) and total cholesterol (2E). - Thickness of line increases with sample size. Line length was defined as ±1 standard deviation (SD) of the study (or overall) mean. Where the mean or SD of a study was not available the average for that analysis was used.
Blood lipids
The summary regression line for total cholesterol (DBS = 0.6807Venous + 1.151) (Figure 2) indicates a requirement for moderate adjustment of values based upon analyses of DBS samples to obtain estimates equivalent to standard analyses based upon venous samples. The regression lines for the two studies contributing to this meta-analysis were directly comparable in terms of both slope and intercept although both were derived from studies done at the same investigational centre. For triglycerides, the summary regression for the three contributing studies showed a close association between the results obtained for the two methods (DBS = 0.9557Venous + 0.1427) (Figure 2) without any evidence of heterogeneity between the three results. Only one data set was available for HDL.
Storage
Data about the circumstances and duration of storage of DBS samples were inconsistently reported with few data to describe whether the analysis findings were affected by extended storage duration or different storage temperatures. From the limited data available it was concluded that DBS samples collected for assay of HbA1c and intended for HPLC analysis can be stored for 5 days at room temperature or for up to 3 years at −70°C [9, 11, 23]. If analysis by immunoturbidimetry is planned, the data variously suggest that samples can be stored safely at room temperature for up to 44 days [10], at 4°C for up to 15 days [25] and storage at −80°C for up to 3 months [33]. For total cholesterol samples were reported as stable for up to 1 month at room temperature [30, 32] and up to 3 months at 4°C [32], and for triglycerides up to 1 month at room temperature and up to 2 months at 4°C [30].
Discussion and conclusion
These analyses identified clear associations between assay results based upon blood samples collected using traditional venous approaches and blood samples collected using DBS techniques, for both HbA1c and selected blood lipids. The data provide a strong rationale for the further investigation of DBS sample collection techniques although also serve to highlight a number of areas that require further exploration before the method is considered mainstream in this field. If, however, standards and calibrations can be agreed, as has been achieved in other fields of research [23, 31, 37], the DBS method does appear to have significant potential to address the logistical challenges of venous sampling for studies of metabolic risks in resource poor settings [17].
The differences between the intercepts of the regression lines obtained for the various analytic methods used for assay of HbA1c require careful consideration in terms of their implications. If the variation is due to the analytic method selected then it will be necessary to recommend a standard approach for each analyte of interest. However, while the analytic method is the obvious explanation for the observed variation it is not possible to exclude alternative causes on the basis of the available data. For example, other aspects of the preparation of the DBS samples such as transportation and extraction were not standardised across the different analytic methodologies and might also be a cause of the differences noted.
The incomplete and summary nature of the data available for analysis placed significant constraints upon the extent to which the results could be explored in this overview. In particular, measures of variance of the data were unavailable for most contributing studies, requiring that weighting be done by sample size alone [18] with consequent limitations upon the methodologies that could be used to present uncertainty intervals around both the individual studies and the summary estimates. For example, we identified a possible relationship between the regression parameters and the mean HbA1c of the contributing studies suggesting that both the intercept and the slope might change when HbA1c rises above 8% (Figures 3 and 4). This implies a non-linear association of venous with DBS sample results that might require a more nuanced explanation than the simple linear regressions provided here [9]. Removing the studies with high average HbA1c levels from the meta-analysis resulted in a regression line approaching parity (DBS = 0.9553 V + 0.2566) and with a reduced heterogeneity for both the slopes (I2 = 0%) and the intercepts (I2 = 92%). However, whether this simply reflects a chance finding in the data, or a true variation of the association by mean HbA1c level is still uncertain. Likewise, several studies used the same patients for two rounds of analysis [21, 28] and there would therefore have been some correlation between the findings for each. This would not be expected to substantially change the parameter estimates obtained but certainly would increase the uncertainty around them. Our inability to create robust uncertainty intervals around our estimates is the primary weakness of this piece of work. Unfortunately the lipid analyses were even less rigorous, with so few studies, confidence in the meta-analysis is limited. There is much potential for further work in this area to generate reliable regression analyses for use in the field.
On a more positive note, the association between results based on venous and DBS samples appeared to be consistent at the levels of HbA1c at which diabetes mellitus is diagnosed (HbA1c > 6.5% [38]). This implies that DBS samples could already be used for determining the presence or absence of diabetes with reasonable certainty, although measures of the extent to which blood glucose is controlled amongst those with diabetes would be less reliable.
Most of the studies reported some information about DBS sample preparation, transport and storage but the data were provided in diverse formats and were substantively incomplete. While it appears likely that DBS samples are stable for adequate periods of time this is an area that requires systematic evaluation and the development of standardised recommendations prior to widespread roll out of the methodology.
The establishment of World Health Organization ‘’25 by 25” target for the prevention of non-communicable diseases [39] has added urgency to the need for data about the metabolic determinants of cardiovascular risk. With more than three quarters of all chronic disease now occurring in developing country settings, the introduction of low cost research techniques that will provide the data required to inform government decision making is a priority [40]. DBS sample collection methods appear to have great potential for the evaluation of cardiometabolic risk factors at the population level [9, 13, 27] enabling data collection at scale in areas previously unstudied [15]. There remain, however, important advances to be made in defining standard methodologies and adjustments before the DBS sampling method is confirmed as a sound proxy for traditional venepuncture samples for these types of blood analytes.
Abbreviations
- DBS:
-
Dried blood spot
- HbA1c:
-
Haemoglobin A1c
- HIV:
-
Human immunodeficiency virus
- HDL:
-
High density lipoprotein
- LDL:
-
Low density lipoprotein
- HPLC:
-
High performance liquid chromatography.
References
Reddy KS: Cardiovascular disease in non-Western countries. N Engl J Med. 2004, 350 (24): 2438-2440. 10.1056/NEJMp048024.
Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M, Baxter A, Bell ML: Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990?2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012, 380 (9859): 2197-2223. 10.1016/S0140-6736(12)61689-4.
Nabel EG, Braunwald E: A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012, 366 (1): 54-63. 10.1056/NEJMra1112570.
Fontbonne A, Eschwege E, Cambien F, Richard JL, Ducimetiere P, Thibult N, Warnet JM, Claude JR, Rosselin GE: Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia. 1989, 32 (5): 300-304. 10.1007/BF00265546.
Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, Tyroler HA: Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990, 322 (24): 1700-1707. 10.1056/NEJM199006143222403.
Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Br Med J. 2000, 321 (7258): 405-412. 10.1136/bmj.321.7258.405.
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Bangdiwala S, Tyroler HA: High-density lipoprotein cholesterol and cardiovascular disease. Four Prospective American Studies Circulation. 1989, 79 (1): 8-15.
Rifkind BM: Lipid research clinics coronary primary prevention trial: results and implications. Am J Cardiol. 1984, 54 (5): 30C-34C. 10.1016/0002-9149(84)90854-3.
Egier DA, Keys JL, Hall SK, McQueen MJ: Measurement of hemoglobin A1c from filter papers for population-based studies. Clin Chem. 2011, 57 (4): 577-585. 10.1373/clinchem.2010.156380.
Jones TG, Warber KD, Roberts BD: Analysis of hemoglobin a1c from dried blood spot samples with the tina-quant® ii immunoturbidimetric method. J Diabetes Sci Technol. 2010, 4 (2): 244-249. 10.1177/193229681000400203.
McDade TW, Williams S, Snodgrass JJ: What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007, 44 (4): 899-925. 10.1353/dem.2007.0038.
Mei JV, Alexander JR, Adam BW, Hannon WH: Use of filter paper for the collection and analysis of human whole blood specimens. Journal Nutrition. 2001, 131 (5): 1631S-1636S.
Parker SP, Cubitt WD: The use of the dried blood spot sample in epidemiological studies. J Clin Pathol. 1999, 52: 633-639. 10.1136/jcp.52.9.633.
Worthman CM, Stallings JF: Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol. 1997, 104 (1): 1-21. 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V.
Bhatti P, Kampa D, Alexander BH, McClure C, Ringer D, Doody MM, Sigurdson AJ: Blood spots as an alternative to whole blood collection and the effect of a small monetary incentive to increase participation in genetic association studies. BMC Medical Res Methodology. 2009, 9: 76-10.1186/1471-2288-9-76.
Wassilak SG, Bernier RH, Herrmann KL, Orenstein WA, Bart KJ, Amler R: Measles seroconfirmation using dried capillary blood specimens in filter paper. Pediatr Infect Dis. 1984, 3 (2): 117-121. 10.1097/00006454-198403000-00007.
Hamers RL, Smit PW, Stevens W, Schuurman R, de Wit TF R: Dried fluid spots for HIV type-1 viral load and resistance genotyping: a systematic review (Structured abstract). Antiviral Therapy. 2009, 15 (5): 619-629.
Becker BJW: The Synthesis of regression slopes in meta-analysis. Statistical Science. 2007, 22 (3): 414-429. 10.1214/07-STS243.
Anjali A, Geethanjali F, Kumar RS, Seshadri M: Accuracy of filter paper method for measuring glycated hemoglobin. J Assoc Physicians India. 2007, 55: 115-119.
Jeppsson JO: Determination of HbA(1c) by the Tina-quant(registered trademark) (a) HbA(1c) immunoassay using dried capillary blood on filter paper. Klinisches Labor. 1993, 39 (12): 1080-1082.
Fokkema MR, Bakker AJ, de Boer F, Kooistra J, de Vries S, Wolthuis A: HbA1c measurements from dried blood spots: validation and patient satisfaction. Clin Chem Lab Med. 2009, 47 (10): 1259-1264.
Gay EC, Cruickshanks KJ, Chase HP, Klingensmith G, Hamman RF: Accuracy of a filter paper method for measuring glycosylated hemoglobin. Diabetes Care. 1992, 15 (1): 108-110. 10.2337/diacare.15.1.108.
Jeppsson JO, Jerntorp P, Almer LO, Persson R, Ekberg G, Sundkvist G: Capillary blood on filter paper for determination of HbA1c by ion exchange chromatography. Diabetes Care. 1996, 19 (2): 142-145. 10.2337/diacare.19.2.142.
Lacher DA, Berman LE, Chen TC, Porter KS: Comparison of dried blood spot to venous methods for hemoglobin A1c, glucose, total cholesterol, high-density lipoprotein cholesterol, and C-reactive protein. Clin Chim Acta. 2013, 422: 54-58.
Lakshmy R, Gupta R: Measurement of glycated hemoglobin A1c from dried blood by turbidimetric immunoassay. J Diabetes Sci Technol. 2009, 3 (5): 1203-1206. 10.1177/193229680900300527.
Little RR, McKenzie EM, Wiedmeyer HM, England JD, Goldstein DE: Collection of blood on filter paper for measurement of glycated hemoglobin by affinity chromatography. Clin Chem. 1986, 32 (5): 869-871.
Lomeo A, Bolner A, Scattolo N, Guzzo P, Amadori F, Sartori S, Lomeo L: HPLC analysis of HbA1c in dried blood spot samples (DBS): a reliable future for diabetes monitoring. Clin Lab. 2008, 54 (5–6): 161-167.
Tabatabaei-Malazy O, Heshmat R, Omidfar K, Pasalar P, Delavari A, Keshtkar A, Larijani B: Glycated hemoglobin measurements from dried blood spots: reliability and relation to results obtained from whole blood samples. J Diabetes Metabolic Disorders. 2011, 10: 1-6.
Wikblad K, Smide B, Bergstrom A, Wahren L, Mugusi F, Jeppsson JO: Immediate assessment of HbA1c under field conditions in Tanzania. Diabetes Res Clin Pract. 1998, 40 (2): 123-128. 10.1016/S0168-8227(98)00046-1.
Lakshmy R, Gupta R, Prabhakaran D, Snehi U, Reddy KS: Utility of dried blood spots for measurement of cholesterol and triglycerides in a surveillance study. J Diabetes Sci Technol. 2010, 4 (2): 258-262. 10.1177/193229681000400206.
Lakshmy R, Mathur P, Gupta R, Shah B, Anand K, Mohan V, Desai NG, Mahanta J, Joshi PP, Thankappan KR: Measurement of cholesterol and triglycerides from a dried blood spot in an Indian Council of Medical Research-World Health Organization multicentric survey on risk factors for noncommunicable diseases in India. J Clin Lipidol. 2012, 6 (1): 33-41. 10.1016/j.jacl.2011.10.021.
Quraishi R, Lakshmy R, Prabhakaran D, Mukhopadhyay AK, Jailkhani B: Use of filter paper stored dried blood for measurement of triglycerides. Lipids Health Disease. 2006, 5 (20): 20-
Buxton OM, Malarick K, Wang W, Seeman T: Changes in Dried Blood Spot HbA1c with Varied Postcollection Conditions. Clin Chem. 2009, 55 (5): 1034-1046. 10.1373/clinchem.2008.111641.
Kapur S, Kapur S, Zava D: Cardiometabolic risk factors assessed by a finger stick dried blood spot method. J Diabetes Sci Technol. 2008, 2 (2): 236-241. 10.1177/193229680800200210.
Slemenda CW, Marrero DG, Fineberg SE, Moore PS, Gibson R: Mail-in paper strip vs microcolumn technique for measurement of glycosylated hemoglobin. Diabetes Care. 1990, 13 (8): 886-888. 10.2337/diacare.13.8.886.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6 (7): e1000097-10.1371/journal.pmed.1000097.
Bruns DE: Standardization, calibration, and the care of diabetic patients. Clin Chem. 1992, 38 (12): 2363-2364.
International Expert C: International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009, 32 (7): 1327-1334.
The NCD Alliance: Final Comprehensive Global Monitoring Framework for NCDs. [http://ncdalliance.org/sites/default/files/rfiles/GMF_Final%20Table%20of%20Targets.pdf]. Accessed May 2014.
Keeling A: DRCP - Agreement of global targets heralds major progress in the fight against diabetes and noncommunicable diseases. Diabetes Res Clin Pract. 2013, 99 (1): 69-70. 10.1016/j.diabres.2012.11.014.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6890/14/21/prepub
Acknowledgements
Bruce Neal is supported by an Australian Research Council Future Fellowship (DP100100295) and a National Health and Medical Research Council of Australia Senior Research Fellowship (APP100311).
Clara Chow is supported by a National Health and Medical Research Council of Australia Career Development Fellowship (APP1033478 ) co-funded by the Heart Foundation and a Sydney Medical Foundation Chapman Fellowship.
D Praveen is supported by the Australian Agency for International Development (AusAID).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
There are no conflicts of interest to be declared.
Authors’ contributions
EA drafted the manuscript and researched data. PD researched data. CC reviewed/edited the manuscript. BN contributed to the study design and re-drafting of the manuscript. Guarantors are EA and BN. All authors’ read and approved the final manuscript.
Electronic supplementary material
12907_2013_166_MOESM1_ESM.pdf
Additional file 1: Figure S1: Funnel plot of the HbA1c regression coefficients. Figure S2. Funnel plot of the HbA1c regression intercepts. (PDF 196 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
About this article
Cite this article
Affan, E.T., Praveen, D., Chow, C.K. et al. Comparability of HbA1c and lipids measured with dried blood spot versus venous samples: a systematic review and meta-analysis. BMC Clin Pathol 14, 21 (2014). https://doi.org/10.1186/1472-6890-14-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6890-14-21