Abstract
Background
Patients may acquire ventilator-associated pneumonia (VAP) by aspirating the condensate that originates in the ventilator circuit upon use of a conventional humidifier. The bacteria that colonize the patients themselves can proliferate in the condensate and then return to the airways and lungs when the patient aspirates this contaminated material. Therefore, the use of HME might contribute to preventing pneumonia and lowering the VAP incidence. The aim of this study was to evaluate how the use of HME impacts the probability of VAP occurrence in critically ill patients.
Methods
On the basis of the acronym "PICO" (Patient, Intervention, Comparison, Outcome), the question that guided this review was "Do critically ill patients under invasive mechanical ventilation present lower VAP incidence when they use HME as compared with HH?". Two of the authors of this review searched the databases PUBMED/Medline, The Cochrane Library, and Latin-American and Caribbean Literature in Health Sciences, LILACS independently; they used the following keywords: "heat and moisture exchanger", AND "heated humidifier", AND "ventilator-associated pneumonia prevention". This review included papers in the English language published from January 1990 to December 2012.
Results
This review included ten studies. Comparison between the use of HME and HH did not reveal any differences in terms of VAP occurrence (OR = 0.998; 95% CI: 0.778–1.281). Together, the ten studies corresponded to a total sample of 1077 and 953 patients in the HME and HH groups, respectively; heterogeneity among the investigations was low (I2 < 50%). Information about the outcome mortality was available in only eight of the ten studies. The use of HME and HH did not afford different results in terms of mortality (OR = 1.09; 95% CI: 0.864–1.376). The total sample size was 884 and 762 patients, respectively. Heterogeneity among the studies was low (I2 = 0.0%).
Conclusion
Current meta-analysis was not sufficient to definitely exclude an associate between heat and moisture exchangers and VAP. Despite the methodological limitations found in selected clinical trials, the current meta-analysis suggests that HME does not decrease VAP incidence or mortality in critically ill patients.
Similar content being viewed by others
Background
Nosocomial pneumonia remains to be one of the main causes of infection in intensive care units (ICU) [1, 2]. This condition is associated with the length of hospital stay [1, 3], duration of mechanical ventilation, and use of broad-spectrum antibiotics. In the particular case of Brazil, nosocomial pneumonia is the primary cause of infection among critically ill patients admitted to the ICU, which is associated with increased hospital costs [1, 2, 4–6] and mortality [3, 6]. This type of pneumonia occurs more frequently in patients submitted to mechanical ventilation for over 48 h, so it is commonly designated ventilator-associated pneumonia (VAP) [7]. VAP diagnosis relies on the emergence of a new pulmonary infiltrate or the presence of progressive pulmonary filtrate accompanied by fever, leukocytosis, and purulent secretion [7]. Mortality due to VAP varies between 24 and 50% and may reach rates as high as 76% in patients with comorbidities such as COPD, diabetes and other chronic lung diseases [8].
Mechanical ventilation suppresses the natural mechanisms that moisturize and heat inhaled air. When patients use an artificial airway, it is necessary to couple the ventilation system with a device that compensates for this suppression [9]. The lack of adequate moisturizing may thicken the secretions, which augments resistance to the passage of air, reduces the gas exchange effectiveness, and increases the risk of respiratory infections [10]. Air moisturizing and heating can be achieved actively (through the use of heated humidifiers, HH) or passively (by means of heat and moisture exchangers, HME) [1].
Patients may acquire VAP by aspirating the condensation of water (ie, overhumidification) that originates in the ventilator circuit upon use of a conventional humidifier. The bacteria that colonize the patients themselves can proliferate in the condensate and then return to the airways and lungs when the patient aspirates this contaminated material [10–12]. Therefore, the use of HME might contribute to preventing pneumonia and lowering the VAP incidence.
Many research papers have compared HME and HH in terms of VAP occurrence [13–17]. However, data about how effectively HME prevents VAP remain inconclusive. Hence, this study aimed to evaluate how the use of HME impacts the probability of VAP occurrence in critically ill patients.
Methods
Data sources and search strategies
This is a systematic review of the literature with meta-analysis. The methodology involved six stages: selection of the hypothesis, selection of the studies, definition of the characteristics, analysis of the studies included in this revision, interpretation of the results, and synthesis of the results.
On the basis of the acronym "PICO" (Patient, Intervention, Comparison, Outcome), the question that guided this review was "Do critically ill patients under invasive mechanical ventilation present lower VAP incidence when they use HME as compared with HH?". Two of the authors of this review searched the databases PUBMED/Medline, The Cochrane Library, and Latin-American and Caribbean Literature in Health Sciences, LILACS independently; they used the following keywords: "heat and moisture exchanger", AND "heated humidifier", AND "ventilator-associated pneumonia prevention". Table 1 presents the strategy these authors used to search for scientific evidence in the databases. This review included papers in the English language published from January 1990 to December 2012.
Study selection
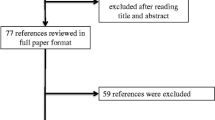
This review only considered controlled randomized clinical assays that evaluated the use of HME as compared with HH to prevent VAP in critically ill patients. Exclusion criteria included: studies that did not associate the use of exchanger with VAP, experimental (non-clinical) studies, economic assessments and reviews, and articles that were not fully available. The following information was relevant for data collection: (1) paper identification (paper and journal title, main author, year of publication, and study locations); (2) assessment criteria used in the studies (exchanger type); (3) methodological characteristics [study type, study aims, results (impact of the exchanger on VAP prevention and possible complications), limitations, and conclusions]. The final sample consisted of 10 articles. Figure 1 illustrates the inclusion process.
Data analysis and statistical methods
The model to assess the quality of clinical assays proposed by the Oxford Centre for Evidence-based Medicine–Levels of Evidence (2009) helped to evaluate the quality of the evidence. Meta-analysis considered two outcomes, namely VAP occurrence and mortality rate among patients belonging to the HME (Intervention A) and the HH (Intervention B) groups, which meant that the outcomes were dichotomous. Odds Ratio (OR) and its 95% Confidence Interval (95% CI) were used for the results of each study and for the synthesis. The Cochran’s Q and the I-square (I 2) tests aided evaluation of heterogeneity. All the data were reported considering 95% CI and two-tailed p values. The random effects model was employed in the case of significant heterogeneity among the studies (I 2 > 50). The fixed effects model was applied for non-significant heterogeneity (I 2 < 50). The meta-analysis was graphically represented by a Forest plot; the publication bias will be represented by a funnel plot. The software Comprehensive Meta Analysis™ version 2.2.064 (CMA Inc. USA) was employed to analyze and record data.
Results
This review included ten studies. Table 2 summarizes the main results and depicts the characteristics of each publication in detail, including the level of evidence provided by each study.
Table 3 compares the VAP incidence and mortality in the different clinical assays.Meta-analysis provided a synthesis of the results considering the VAP outcome in the ten clinical assays selected for this review (Figure 2).
Comparison between the use of HME and HH, in this meta-analysis did not reveal any differences in terms of VAP occurrence (OR = 0.998; 95% CI: 0.778–1.281). Together, the ten studies corresponded to a total sample of 1077 and 953 patients in the HME and HH groups, respectively; heterogeneity among the investigations was low (I2 < 50%).Information about the outcome mortality was available in only eight of the ten studies. Hence, the results synthesis can be seen in the referent meta-analysis (Figure 3).
In this meta-analysis the use of HME and HH was not associated with different rates/risk of mortality (OR = 1.09; 95% CI: 0.864–1.376). The total sample size was 884 and 762 patients, respectively. Heterogeneity among the studies was low (I2 = 0.0%).Considering the two outcomes investigated here, Figure 4 does not evidence any publication bias.
Discussion
Literature studies have presented conflicting results about how HME impacts VAP prevention. A meta-analysis including nine studies and 1368 patients revealed that HME reduced VAP rates especially in the case of subjects submitted to mechanical ventilation for over seven days (RR = 0.7; IC 95%: 0.50-0.94). However, non-randomized studies (not included in this meta-analysis) found significantly lower VAP rates in the groups that used HH as compared with HME [18]. Other two randomized studies reported non-significantly different VAP rates for HH and HME [14, 15]. A randomized study of 120 patients demonstrated smaller VAP incidence during the use of HH in patients under mechanical ventilation for periods longer than five days (15.69 vs 39.62%, P = 0.006) [19]. A meta-analysis published by Siempos et al. (2007) [16] included 13 randomized clinical assays and 2,580 patients; it did not detect any differences between HME and HH with respect to VAP incidence, mortality in the ICU, length of stay in the ICU, period of mechanical ventilation, or airway obstruction.
Most studies have found similar VAP rates for HME and HH [14–18, 20–26]. In most of these studies, the HME and HH groups did not differ in terms of VAP incidence density, period of mechanical ventilation, length of stay in the ICU, or global mortality rate.
Kirton et al. (1997) [24] evaluated 280 trauma patients and demonstrated lower VAP incidence during the use of HME. On the other hand, Auxiliadora-Martins et al. (2012) [17] investigated severely ill patients (mean APACHE II score > 25) with associated comorbidities, to find that the HH and HME group did not have different VAP incidence. The heterogeneous study populations, the distinct brands of HME employed in the aforementioned studies, the frequency with which the nursing staff changed the moisturizer, and the criteria used to diagnose VAP may have contributed to these contrasting results [19]. Nevertheless, HME did offer advantages over HH: it did not require that the staff opened the device circuit to remove the condensate accumulated in the ventilator extensions, which reduced the occupational risk and optimized the work of the nursing team. In addition, it may have some disadvantages using HH; such as high maintenance costs and mechanical malfunction of the humidification and mechanical ventilator apparatus, and overheating of the inspired gases. These problems could be avoided by using HME [10–12, 17].
Even though many studies have shown that the use of HME incurs lower costs [14, 15, 21, 22, 24, 25], this device is not risk-free. Partial or total tube occlusion is the most probable adverse effect, and its frequency is significantly different in patients submitted to HME and HH [14, 22, 23, 26]. Lower moisture production may account for this difference, especially during application of larger minute volumes. This is a common event in all the commercially available devices, albeit to different extent [27–29]. Martin et al. (1990) [23] have also described more frequent cases of hypothermia in patients using HME. In contrast, Kirton et al. (1997) [24] and Kollef et al. (1998) [25] did not observe any differences between the HH and HME groups in terms of tube occlusion. The use of HME involves other concerns: increased dead volume due to hypercapnia and larger airway resistance, which depend mainly on the filter internal volume and liquid accumulation, respectively [29–31].
In most studies the change of HME was every 24 or 48 hours according manufacturer’s recommendation. Other studies have demonstrated that prolonged use of HME, from 24–48 h up to four or seven days, does not incur increased risk of VAP [31–35]. Davis et al. did not detect reduced efficiency, increased resistance, or altered bacterial colonization when the use of HME was discontinued after three days. The VAP incidence rate did not change, either, so the authors concluded that the use of HME for over 24 h, up to 72 h, is safe and economically advantageous.
Together, the analyzed studies suggest that it is possible to use either HH or HME without significantly impacting the VAP incidence. Considering hospital costs and in the absence of contraindications, HME should be employed as an alternative humidifier in patients submitted to mechanical ventilation. The results of the present review agree with the pathophysiology proposed for VAP in the sense that bacteria inoculation into the lungs generally occurs via extraluminal source, whereas only occasionally does the intraluminal pathway happen.
Considering a 30% reduction in the occurrence of VAP among patients who used respectively, HH (16%) and HME (11%), with a significance level of 5% and a statistical power of 80%, the minimum sample per group would be 731 subjects. In the present meta-analysis, it was evaluated studies with 953 subjects in the HH group and 1077 in the HME group, thus this review presents a sufficient sample size to detect this difference. However there are some potential limitations should be considered. Firstly, the sample size was sufficient to detect a 30% reduction in VAP rates; the power of the study was not sufficient to identify more modest reductions, although smaller reductions could still be important from a clinical perspective. Secondly, some studies were not calculated sample size and were not described the randomization procedure. Thirdly, in two studies patients were excluded after randomization and other two studies were non blinded. Finally, the definitions of VAP varied in some included studies. Thus other double-blind studies, randomized controlled should be performed to definitely exclude an associate between heat and moisture exchangers and VAP.
Hence, the studies included in this meta-analysis did not attest that the use of HME in the ICU setting reduced the VAP incidence or affected mortality rates. On the basis of these two outcomes (VAP incidence and mortality), the meta-analysis of the selected studies was reliable, given that the samples had low heterogeneity.
Conclusion
Current meta-analysis was not sufficient to definitely exclude an associate between heat and moisture exchangers and VAP. Despite the methodological limitations found in selected clinical trials, the current meta-analysis suggests, with some degree of uncertainty, that HME does not decrease VAP incidence or mortality in critically ill patients and therefore, further clinical trials with greater methodological rigor and adequate sample size should be performed comparing HH to HME in the incidence of VAP and other important outcomes such as mortality. Is important to highlight that, institutions routinely using HME should be aware of obstruction events and cases of hypercapnia and hypothermia, because some studies have described their occurrence.
Consent
Written informed consent was obtained from the patient for the publication of this report.
References
Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M: The prevalence of nosocomial infection in intensive care units in Europe. Results of the European prevalence of infection in intensive care (EPIC) study. EPIC international advisory committee. JAMA. 1995, 274: 639-644. 10.1001/jama.1995.03530080055041.
Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH: Epidemiology and outcomes of ventilator associated pneumonia in a large US database. Chest. 2002, 122: 2115-2121. 10.1378/chest.122.6.2115.
Bercault N, Boulain T: Mortality rate attributable to ventilator associated nosocomial pneumonia in an adult intensive care unit: a prospective case–control study. Crit Care Med. 2001, 29: 2303-2309. 10.1097/00003246-200112000-00012.
Chen YY, Wang FD, Liu CY, Chou P: Incidence rate and variable cost of nosocomial infections in different types of intensive care units. Infect Control Hosp Epidemiol. 2009, 30: 39-46. 10.1086/592984.
Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, Cohen MM, Fraser VJ: Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003, 31: 1312-1317. 10.1097/01.CCM.0000063087.93157.06.
Safdar N, Dezfulian C, Collard HR, Saint S: Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005, 33: 2184-2193. 10.1097/01.CCM.0000181731.53912.D9.
Horan TC, Andrus M, Dudeck MA: CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008, 36: 309-332. 10.1016/j.ajic.2008.03.002.
Meduri GU: Diagnosis and differential diagnosis of ventilator associated pneumonia. Clin Chest Med. 1995, 16: 61-93.
Branson RD, Chatburn RL: Humidification of inspired gases during mechanical ventilation. Respir Care. 1993, 38: 461-468.
Hess DR, Kacmarek RM: Essentials of Mechanical Ventilation. 1996, New York: McGraw-Hill, 33-40.
Craven DE, Goularte TA, Make RJ: Contaminated condensate in mechanical ventilator circuits: a risk factor for nosocomial pneumonia?. Am Rev Respir Dis. 1984, 129: 625-628.
Martin C, Thomachot L, Quinio R, Viviand X, Albanese J: Comparing two heat and moisture exchangers with one vaporizing humidifier in patients with minute ventilation greater than 10 L/min. Chest. 1995, 107: 1411-1415. 10.1378/chest.107.5.1411.
Kola A, Eckmanns T, Gastmeier P: Efficacy of heat and moisture exchangers in preventing ventilator-associated pneumonia: meta-analysis of randomized controlled trials. Intensive Care Med. 2005, 31: 5-11. 10.1007/s00134-004-2431-1.
Lacherade JC, Auburtin M, Cerf C, Van de Louw A, Soufir L, Rebufat Y, Rezaiguia S, Ricard JD, Lellouche F, Brun-Buisson C, Brochard L: Impact of humidification systems on ventilator-associated pneumonia: a randomized multicenter trial. Am J Respir Crit Care Med. 2005, 172: 1276-1282. 10.1164/rccm.200408-1028OC.
Boots RJ, George N, Faoagali JL, Druery J, Dean K, Heller RF: Double-heater-wire circuits and heat-and-moisture exchangers and the risk of ventilator-associated pneumonia. Crit Care Med. 2006, 34: 687-693. 10.1097/01.CCM.0000201887.51076.31.
Siempos II, Vardakas KZ, Kopterides P, Falagas ME: Impact of passive humidification on clinical outcomes of mechanically ventilated patients: a meta-analysis of randomized controlled trials. Crit Care Med. 2007, 35: 2843-2851. 10.1097/01.CCM.0000295302.67973.9A.
Auxiliadora-Martins M, Menegueti MG, Nicolini EA, Alkmim-Teixeira GC, Bellissimo-Rodrigues F, Martins-Filho OA, Basile-Filho A: Effect of heat and moisture exchangers on the prevention of ventilator-associated pneumonia in critically ill patients. Braz J Med Biol Res. 2012, 45 (12): 1295-1300. 10.1590/S0100-879X2012007500161.
Bench S: Humidification in the long-term ventilated patient; a systematic review. Intensive Crit Care Nurs. 2003, 19 (2): 75-84. 10.1016/S0964-3397(03)00024-7.
Lorente L, Lecuona M, Jimenez A, Mora ML, Sierra A: Ventilator-associated pneumonia using a heated humidifier or a heat and moisture exchanger: a randomized controlled trial [ISRCTN88724583]. Crit Care. 2006, 10: R116-10.1186/cc5009.
Memish ZA, Oni GA, Djazmati W, Cunningham G, Mah MW: A randomized clinical trial to compare the effects of a heat and moisture exchanger with a heated humidifying system on the occurrence rate of ventilator-associated pneumonia. Am J Infect Control. 2001, 29: 301-305. 10.1067/mic.2001.115404.
Boots RJ, Howe S, George N, Harris FM, Faoagali J: Clinical utility of hygroscopic heat and moisture exchangers in intensive care patients. Crit Care Med. 1997, 25: 1707-1712. 10.1097/00003246-199710000-00021.
Dreyfuss D, Djedaini K, Gros I, Mier L, Le Bourdelles G, Cohen Y, Estagnasié P, Coste F, Boussougant Y: Mechanical ventilation with heated humidifiers or heat and moisture exchangers: effects on patient colonization and incidence of nosocomial pneumonia. Am J Respir Crit Care Med. 1995, 151: 986-992.
Martin C, Perrin G, Gevaudan MJ, Saux P, Gouin F: Heat and moisture exchangers and vaporizing humidifiers in the intensive care unit. Chest. 1990, 97: 144-149. 10.1378/chest.97.1.144.
Kirton OC, DeHaven B, Morgan J, Morejon O, Civetta J: A prospective, randomized comparison of an in-line heat moisture exchange filter and heated wire humidifiers: rates of ventilator-associated early-onset (community-acquired) or late-onset (hospital-acquired) pneumonia and incidence of endotracheal tube occlusion. Chest. 1997, 112: 1055-1059. 10.1378/chest.112.4.1055.
Kollef MH, Shapiro SD, Boyd V, Silver P, Von Harz B, Trovillion E, Prentice D: A randomized clinical trial comparing an extended-use hygroscopic condenser humidifier with heated-water humidification in mechanically ventilated patients. Chest. 1998, 113: 759-767. 10.1378/chest.113.3.759.
Roustan JP, Kienlen J, Aubas P, Aubas S, du Cailar J: Comparison of hydrophobic heat and moisture exchangers with heated humidifier during prolonged mechanical ventilation. Intensive Care Med. 1992, 18: 97-100. 10.1007/BF01705040.
Cohen IL, Weinberg PF, Alan I, Rowinsky GS: Endotracheal tube occlusion associated with the use of heat and moisture exchangers in the intensive care unit. Crit Care Med. 1988, 16: 277-279. 10.1097/00003246-198803000-00013.
Doyle A, Joshi M, Frank P, Craven T, Moondi P, Young P: A change in humidification system can eliminate endotracheal tube occlusion. J Crit Care. 2011, 26 (6): 637-
Hurni JM, Feihl F, Lazor R, Leuenberger P, Perret C: Safety of combined heat and moisture exchanger filters in long-term mechanical ventilation. Chest. 1997, 111: 686-691. 10.1378/chest.111.3.686.
Briassoulis G, Paraschou D, Hatzis T: Hypercapnia due to a heat and moisture exchanger. Intensive Care Med. 2000, 26: 147-10.1007/s001340050033.
Wilkes AR: Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 1 - history, principles and efficiency. Anaesthesia. 2011, 66: 31-39. 10.1111/j.1365-2044.2010.06563.x.
Wilkes AR: Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 2 - practical use, including problems, and their use with paediatric patients. Anaesthesia. 2011, 66: 40-51.
Markowicz P, Ricard JD, Dreyfuss D, Mier L, Brun P, Coste F, Boussougant Y, Djedaïni K: Safety, efficacy, and cost-effectiveness of mechanical ventilation with humidifying filters changed every 48 hours: a prospective, randomized study. Crit Care Med. 2000, 28: 665-671. 10.1097/00003246-200003000-00011.
Thomachot L, Boisson C, Arnaud S, Michelet P, Cambon S, Martin C: Changing heat and moisture exchangers after 96 hours rather than after 24 hours: a clinical and microbiological evaluation. Crit Care Med. 2000, 28: 714-720. 10.1097/00003246-200003000-00019.
Thomachot L, Leone M, Razzouk K, Antonini F, Vialet R, Martin C: Randomized clinical trial of extended use of a hydrophobic condenser humidifier: 1 vs. 7 days. Crit Care Med. 2002, 30: 232-237. 10.1097/00003246-200201000-00033.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2253/14/115/prepub
Acknowledgments
We are thankful to Fundação de Amparo ao Ensino, Pesquisa e Assistência (FAEPA) do Hospital das Clínicas and Faculdade de Medicina de Ribeirão Preto-USP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MGM, contributed with data analysis and interpretation. AAN, contributed with statistical analysis. MA-M and MGM, contributed with drafting the article. MGM, MAM and AAN contributed with revising it critically for important intellectual content. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Menegueti, M.G., Auxiliadora-Martins, M. & Nunes, A.A. Effectiveness of heat and moisture exchangers in preventing ventilator-associated pneumonia in critically ill patients: a meta-analysis. BMC Anesthesiol 14, 115 (2014). https://doi.org/10.1186/1471-2253-14-115
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2253-14-115