Abstract
Mucosal Schwann cell hamartomas (MSCHs) are recently characterized benign spindle cell lesions of the colon and rectum. There is only one report of MSCHs in the stomach (antrum). Herein, we present the first reports of MSCHs occurring in the fundic mucosa as lesions that endoscopically mimicked a typical fundic gland polyp. Case 1. A 56-year-old woman sought medical attention due to epigastric pain. A small polypoid lesion in the fundic mucosa was removed with an endoscopic impression of a fundic gland polyp. Case 2. A 66-year-old man sought medical attention due to epigastric pain. The patient underwent antral and corporal biopsies that showed mild non-active gastritis without Helicobacter pylori infection. Small polypoid lesions in the fundic mucosa were seen; one was removed with an endoscopic impression of the fundic gland polyp. At the microscope, both lesions were entirely intramucosal, ill-defined spindle cell aggregations, suggesting a fascicular growth pattern. Both lesions were strongly and diffusely positive for S100. Awareness of this lesion is relevant to avoid the diagnosis of other benign spindle cell lesions that are associated with familial syndromes.
Similar content being viewed by others
Introduction
Mucosal Schwann cell hamartomas (MSCHs) have been recently characterized as benign spindle cell lesions of the colon and rectum. These lesions are usually small and incidental findings upon colonoscopy. Awareness of this entity is important to avoid the diagnosis of certain similar lesions, such as mucosal neuromas and neurofibromas, associated with inherited familial syndromes that are associated with neoplasia (Davis and Berk 1973; Fuller and Williams 1991; Hochberg et al. 1974; Lee and Norton 2000; Lewin et al. 2005). To the best of our knowledge, there is only one published report of MSCH in the stomach (Hytiroglou et al. 2016). Herein, we present the first reports of MSCHs occurring in the fundic mucosa in lesions that endoscopically mimicked typical fundic gland polyps.
Case presentation
Case 1
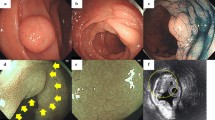
A 56-year-old woman sought medical attention due to epigastric pain. She had no relevant past medical history. The patient underwent antral and corporal biopsies that showed chronic active gastritis associated with Helicobacter pylori infection. A small polypoid lesion in the fundic mucosa was removed with an endoscopic impression of the fundic gland polyp (Fig. 1). After the diagnosis, the patient was re-examined; no features of neurofibromatosis, multiple endocrine neoplasia or Cowden syndrome were clinically detected. After treatment, endoscopy was normal. After the diagnosis, the patient was clinically reevaluated, and a thyroid nodule was detected. A fine-needle aspiration biopsy was performed with a diagnosis of a Bethesda II (benign) lesion, thus excluding the possibility of medullary carcinoma, a feature of multiple endocrine neoplasia. The lesion was an ill-defined spindle cell aggregation, suggesting a fascicular growth pattern. No nuclear atypia was seen. The whole lesion measured 0.6 mm and was entirely intramucosal. The lesion was strongly and diffusely positive for S100 (polyclonal, Agilent, pre-diluted) (Fig. 1) and was negative for smooth muscle actin (1A4, Agilent, pre-diluted), c-KIT/CD117 (polyclonal, Agilent, 1:100), epithelial membrane antigen (E29, Agilent, pre-diluted), CD34 (QBEnd10, Agilent, pre-diluted), synaptophysin (SY38, Agilent, pre-diluted), chromogranin (polyclonal, Agilent, 1:300) and CD56 (123C3, Agilent, pre-diluted). The diagnosis was then reported as MSCHs of the fundic mucosa.
Case 1. Endoscopic appearance of a polypoid lesion in the gastric fundus (a). Mucosal Schwann cell hamartoma in the gastric mucosa. A poorly circumscribed spindle cell lesion with no significant atypia: b 40x magnification; c 100x magnification. Mucosal Schwann cell hamartoma in the gastric mucosa. Strong and diffuse immunohistochemical staining for S100 protein (100x magnification) (d)
Case 2
A 66-year-old man sought medical attention due to epigastric pain. The patient underwent antral and corporal biopsies that showed mild non-active gastritis with no Helicobacter pylori infection. Small polypoid lesions in the fundic mucosa were seen; one was removed with an endoscopic impression of the fundic gland polyp. The patient had an unremarkable medical history; no signs of familial syndromes were observed. The lesion was entirely intramucosal and was observed to be ill-defined spindle cell aggregations permeating preexistent gastric glands. No nuclear atypia was seen. The lesion measured 2.2 mm. Again, the lesion was strongly and diffusely positive for S100 (Fig. 2) and was negative for all other tested markers (described above).
Discussion
MSCHs were described in a series of 26 cases in the colon and rectum by Gibson and Hornick; these 26 cases were compared to five submucosal neurofibromas from patients with known type 1 fibromatosis (Gibson and Hornick 2009). The lesions were small (mean 2.5 mm, ranging from 1 to 6 mm). The mean age at diagnosis was 62 years, and there was a slight female:male predominance of 16:10. Follow up was available for 13 patients (mean 5.5 years); none had signs of type 1 neurofibromatosis (known to be associated with gastrointestinal neurofibromas) (Davis and Berk 1973; Fuller and Williams 1991), multiple endocrine neoplasia 2B (known to be associated with mucosal neuromas) (Lee and Norton 2000) or Cowden syndrome (which may be related to ganglioneuromatous polyposis) (Hizawa et al. 1994; Lashner et al. 1986).
MSCHs are characterized as poorly circumscribed lesions, often showing entrapment of colonic crypts. The lesion is composed of spindle cells with no atypia or immunohistochemical features of Schwann cell differentiation (Gibson and Hornick 2009). All the cases showed strong and diffuse staining for S100 protein; some cases (7/26) showed rare axons highlighted by neurofilament proteins. Other markers such as epithelial membrane antigen (a marker of perineuroma), CD34 and c-KIT (a marker of gastrointestinal stromal tumors) were uniformly negative.
In these cases, differential diagnosis includes other benign appearing spindle cell lesions of the gastrointestinal wall. Many polyps resected in the original description by Gibson and Hornick were originally diagnosed as colonic neurofibromas. Neurofibromas are usually submucosal or deeper lesions and show S100 protein in only part of the lesional cells, they always contain scattered axons and, importantly, they are strongly associated with type 1 neurofibromatosis. Gastrointestinal stromal tumors (GISTs) are also usually submucosal and may be associated with type 1 neurofibromatosis (Miettinen et al. 2006; Miettinen and Lasota 2006). Negative staining for c-Kit in this case is strong evidence against this diagnosis. Mucosal neuromas are rare, are strongly associated with multiple endocrine neoplasia 2B and are usually restricted to the lips and tongue (Lee and Norton 2000; Lewin et al. 2005). Microscopically, mucosal neuromas show hyperplastic bundles of nerve fibers and frequent axons. Ganglioneuromas are associated with Cowden syndrome (Hizawa et al. 1994; Lashner et al. 1986); these lesions have been described in the stomach (Coriat et al. 2011). In the present case, the lack of ganglion cells eliminated ganglioneuroma as the diagnosis. Intramucosal perineuromas have ill-defined margins; however, they are characterized by ovoid shaped cells in a lamellar or whorled architecture and have immunoreactivity to epithelial membrane antigen. Finally, gastrointestinal schwannomas are also benign proliferations of Schwann cells, but they are usually submucosal (or deeper) well circumscribed, and they usually show a peripheral lymphoid cuff with hyperplastic follicles with germinal centers (Kwon et al. 2002) .
Mucosal Schwann cell hamartomas do occur in the gastric mucosa. Awareness of this lesion is relevant to avoid the diagnosis of other benign spindle cell lesions that are associated with familial syndromes.
Abbreviations
- GIST:
-
Gastrointestinal stromal tumors
- MSCH:
-
Mucosal Schwann cell hamartomas
References
Coriat R, Mozer M, Caux F, Chryssostalis A, Terris B, Grandjouan S et al (2011) Endoscopic findings in Cowden syndrome. Endoscopy 43(8):723–726
Davis GB, Berk RN (1973) Intestinal neurofibromas in von Recklinghausen’s disease. Am J Gastroenterol 60(4):410–414
Fuller CE, Williams GT (1991) Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease). Histopathology 19(1):1–11
Gibson JA, Hornick JL (2009) Mucosal Schwann cell “hamartoma”: clinicopathologic study of 26 neural colorectal polyps distinct from neurofibromas and mucosal neuromas. Am J Surg Pathol 33(5):781–787
Hizawa K, Iida M, Matsumoto T, Kohrogi N, Suekane H, Yao T et al (1994) Gastrointestinal manifestations of Cowden’s disease. Report of four cases. J Clin Gastroenterol 18(1):13–18
Hochberg FH, Dasilva AB, Galdabini J, Richardson EP Jr (1974) Gastrointestinal involvement in von Recklinghausen's neurofibromatosis. Neurology 24(12):1144–1151
Hytiroglou P, Petrakis G, Tsimoyiannis EC (2016) Mucosal Schwann cell hamartoma can occur in the stomach and must be distinguished from other spindle cell lesions. Pathol Int 66(4):242–243
Kwon MS, Lee SS, Ahn GH (2002) Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract 198(9):605–613
Lashner BA, Riddell RH, Winans CS (1986) Ganglioneuromatosis of the colon and extensive glycogenic acanthosis in Cowden's disease. Dig Dis Sci 31(2):213–216
Lee NC, Norton JA (2000) Multiple endocrine neoplasia type 2B--genetic basis and clinical expression. Surg Oncol 9(3):111–118
Lewin MR, Dilworth HP, Abu Alfa AK, Epstein JI, Montgomery E (2005) Mucosal benign epithelioid nerve sheath tumors. Am J Surg Pathol 29(10):1310–1315
Miettinen M, Fetsch JF, Sobin LH, Lasota J (2006) Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 30(1):90–96
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23(2):70–83
Acknowledgements
Not applicable.
Availability of data and materials
Data is available upon request.
Author information
Authors and Affiliations
Contributions
MHOC analyzed the slides and critically reviewed the manuscript for important intellectual content; RJF collected clinical data and critically reviewed the manuscript for important intellectual content; AFC collected clinical data and critically reviewed the manuscript for important intellectual content; CLMG critically reviewed the manuscript for important intellectual content, PRFA analyzed the slides and critically reviewed the manuscript for important intellectual content, and DAA analyzed the slides and drafed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Patients gave signed consent for case publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oliveira Castro, M.H., Felipe, R.J., Fernandes Cardoso, A. et al. Mucosal Schwann cell hamartomas do occur the gastric mucosa – report of two cases mimicking fundic gland polyps. Surg Exp Pathol 2, 1 (2019). https://doi.org/10.1186/s42047-018-0026-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-018-0026-3