Abstract
Background
Ecological communities are organized by interactions among the biota, and between the biota and external environmental drivers that affect the dynamics of individual taxa. Climate change may alter communities in unexpected ways when environmental drivers have complex effects on individual species that are then transmitted indirectly to other species via biotic interactions.
Methods
We used a multivariate autoregressive (MAR) modeling framework to assess the strengths of intrinsic interactions and extrinsic (environmental) forcing responsible for changes in the zooplankton community of a sockeye salmon nursery lake in southwestern Alaska from 1963 to 2009. During this time period there has been a strong trend towards earlier spring ice breakup dates and warmer summer water temperatures.
Results
MAR analyses of community time-series showed that water temperature was the dominant driver of change in the zooplankton community; competitive interactions were relatively rare, and only copepods (both cyclopoids and calanoids) were affected by predation (juvenile sockeye salmon). Best-fit community models were used to develop scenarios of zooplankton community composition under several different potential climate conditions and salmon densities and revealed the potential for a shift in the dominant zooplankton taxa in this lake, driven largely by taxon-specific sensitivity to climate and sockeye salmon predation.
Conclusions
Simulations suggest that cladocerans will become more prevalent in this community and that calanoid copepods will suffer from ongoing climate warming. These results have important implications for fish in these northern lakes, as they suggest that the production of planktivorous fish should increase with ongoing climate change.
Similar content being viewed by others
Background
Ecological communities are organized by a combination of intrinsic interactions associated with competition, predation, parasitism and mutualism, and extrinsic interactions associated with environmental effects on individual species [1,2,3]. Predicting climate change effects on populations depends in part on understanding not only the direct effects of changing environmental conditions on species of interest, but also how climate effects are transmitted through food webs via species interactions. Thus, a key to understanding general ecosystem responses to climate change, or understanding the responses of specific ecosystems, is quantifying how changes in environmental conditions translate to population and ecosystem changes via a combination of direct and indirect effects. At present, we have a very limited understanding of climate-driven indirect effects on whole communities in natural ecosystems.
Traditionally, community and food web ecologists have emphasized experimental approaches to understanding interactions within communities, often at relatively small temporal and spatial scales [4, 5]. More recently, ecologists have begun to use statistical time-series models to estimate interaction strengths among species, and between species and the environment, which can be fit to data collected at spatial and temporal scales that appropriately reflect ecosystem-level processes and functioning [6,7,8].
Multivariate autoregressive (MAR) models are a useful tool for estimating the strengths of interspecific interactions and extrinsic forcing responsible for changes in ecological communities [6, 9]. MAR models are based on the classic deterministic Gompertz model of population growth, which describes change in abundance through time as a function of intrinsic growth and density dependence. MAR models expand on the univariate Gompertz model, and can be described as several interrelated multiple regressions (one for each species) carried out with time-lagged data and then solved simultaneously to find the most parsimonious model describing the changes in species abundance as a function of intra- and interspecific interactions within a community and key exogenous drivers [10]. MAR models have been successfully used to investigate the structural features of plankton communities both within and between lakes [10,11,12,13]. MAR models have a broad range of applications and have been used to estimate community stability [10], estimate the direct effects of planktivory on zooplankton communities [11] and nutrients on phytoplankton [14], to assess the effects of temporal scale of observation on community dynamics [12, 15, 16], to understand the response of plankton communities to environmental change on long [17] and short [18] time scales, and to investigate the role of fishing pressure and fish declines on food web dynamics [19, 20].
Plankton communities provide an excellent opportunity to understand how climate effects are transmitted through communities because monitoring plankton communities is relatively simple compared to monitoring communities with wide-ranging species, and because fast turnover rates in plankton allow many generations to be represented in modest-length time series. An analysis of the plankton community of Lake Baikal, for example, showed significant increases in water temperature, substantial increases in cladoceran densities (over 3-fold increase since 1946) and subtle declines in copepods [17, 21]. MAR analysis of this community revealed that temperature effects on species dwarfed the effects of biotic interactions, and that, when biotic interactions were present, the two main taxonomic groups of zooplankton (cladocerans and copepods) interacted differently with various phytoplankton groups, suggesting strong potential for shifts in species dominance to have large effects on the entire aquatic community.
Southwest Alaska, USA, is a region that has experienced some of the fastest warming trends observed across the globe during the last century [22]. Although the extent of change in climate features has been fairly well described for this region [23], the responses of ecological communities to climate change remain poorly understood. Previous work has evaluated changes in zooplankton production rates in response to climate change [24], as well as assessed shifts in fish community composition in response to habitat loss, climate change and commercial fishery management strategies [25]. However, the vast majority of attention in this region has focused on how climate variables interact with density-dependent effects to influence the growth and survival of the juvenile stage of the commercially and culturally important sockeye salmon [26, 27].
Juvenile sockeye salmon (Onchorhynchus nerka) are voracious and effective consumers of zooplankton [28], and their impacts on zooplankton communities have been well established. In Lake Aleknagik, juvenile sockeye salmon are one of the dominant planktivorous predators (along with three-spine sticklebacks) and are present in relatively high abundances [26]. Schindler et al. [26] considered individual zooplankton density responses to climate and predation by juvenile sockeye, determining that seasonal densities of the cladoceran Daphnia, in particular, showed strong positive responses to climate and have supported increased growth of juvenile sockeye in a sockeye nursery lake. Because juvenile sockeye growth is primarily supported by zooplankton, and because environmental effects on species are often mediated by food web interactions, it is critical to assess the response of zooplankton communities to changing climate in the context of biotic interactions within the community, if we are to gain insight on how early life stages of this foundational species might respond to future conditions.
Here we use a 47-year dataset of zooplankton densities and thermal characteristics of Lake Aleknagik, southwest Alaska, previously used to evaluate taxa-specific responses to climate change [24], to assess how changing climate conditions are translated through biological interactions in a zooplankton community. We use the MAR modeling framework to quantify the strength of external drivers and biotic interactions within the community, and to determine if those interactions are modulated by climate factors. Given what we know about freshwater ecosystems, we expect to see results similar to Hampton et al. and Izmest’eva et al. [17, 21] – that the composition of the zooplankton community in Lake Aleknagik will primarily be structured by external climate drivers, with cladocerans being more sensitive to warming than copepods We then use the best-fit community model to develop a variety of potential future climate change and salmon density scenarios to investigate how warming may propagate through the food web.
Methods
Field collections
The Alaska Salmon Program at the University of Washington has conducted ecological research in the Wood River Lakes, near Bristol Bay, Alaska (Fig. 1) since 1946. This lake system has not been subjected to the anthropogenic perturbations (e.g. dams, mines, sewage effluent) that have affected many lakes with long-term datasets, and that complicate direct analysis of the effects of historical climate warming on ecological characteristics of the ecosystem (e.g. [29,30,31,32,33]). This system is comprised of five large lakes that drain into Bristol Bay via the Wood River. These lakes and their tributaries support large populations of anadromous sockeye salmon (Oncorhynchus nerka) as well as several freshwater resident fish species, and smaller populations of other anadromous salmon species. Zooplankton species present in the lakes include the calanoid copepods Eudiaptomus gracilis, Eurytemora yukonensis, and Leptodiaptomus pribilofensis, the cyclopoid copepods Cyclops columbianus and Acanthocyclops brevispinosus and the cladocerans Eubosmina longispina, Daphnia longiremis, and Holopedium gibberum. Zooplankton, particularly calanoid copepods, are currently the main pelagic prey for juvenile sockeye and other juvenile salmonids, as well as the small resident fish in the Wood River System (Schindler, unpublished data).
Lake Aleknagik has a surface area of 83 km2, mean and maximum depths of 43 and 110 m, respectively, and is dimictic, usually being ice free from about June 1 until mid-December, though there has been a general trend towards a longer ice-free season over the course of the time-series examined here. The lake is typically thermally stratified from mid-June through mid-September.
Zooplankton samples were collected from Lake Aleknagik approximately every ten days at six stations arrayed across the length of the lake between June and September from 1963 to 2009. Plankton were sampled with vertical tows of a 247 μm mesh conical net with a 0.5 m diameter and a width:length ratio of 1:3. The net was retrieved at a rate of ~0.5 m/s either by hand or with a gas powered winch. To help ensure tows remained vertical, a 0.8 kg weight was suspended from the bottom of the net. Zooplankton were preserved in either 10% formalin or 50–70% ethanol. Consecutive subsamples from each sample were enumerated under a dissecting microscope until ~500 individual adult zooplankters were counted, and areal densities (#/m2) were calculated from these raw counts, assuming 100% net efficiency. While the species present in the lake are currently known, zooplankton were not enumerated to the species level in the historical data. Historical zooplankton counts exist at the coarse taxonomic level, aggregated based on functional groups rather than abundance — calanoid copepods, cyclopoid copepods, Daphnia, Bosmina, and Holopedium. Because there was variation in the specific sampling dates and sampling frequency over the 47-year dataset, densities were averaged across the six sampling locations, as well as across time, to provide a single monthly estimate for all years. Average monthly zooplankton densities are available as supplementary material (see Additional file 1).
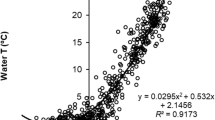
Temperature-depth profiles were collected simultaneously with zooplankton samples using either a digital thermister (Yellow Springs Instruments Inc.) or bathythermograph. To more accurately characterize the thermal environment, water column temperatures were linearly interpolated to every meter and averaged from 0 to 20 m. The average 0–20 m temperature from each station was then averaged across all stations within the lake to give a single lake-wide estimate for each sampling date. All sampling dates within a month were then averaged to give a monthly mean lake temperature (0–20 m) time series.
Adult sockeye salmon spawning densities (escapement) in Lake Aleknagik were estimated from the Alaska Department of Fish and Game (ADF&G) Wood River escapement count and ground and aerial surveys of stream, river and beach spawning habitats in Lake Aleknagik. Adult escapement to Aleknagik in the previous year was used as an index of juvenile sockeye density in the lake for any given year [26], as well as a proxy for marine-derived nutrients. Threespine stickleback abundance is based on townet catch rates from sampling conducted during the last week in August throughout the time series. Townetting consisted of a 3 m × 3 m net towed at the surface between two boats for 5 min at nine standard tow stations [26].
Statistical analysis of climate drivers
We used multivariate autoregressive (MAR) models [6] to describe the strength of interactions among Lake Aleknagik zooplankton, and the effects of environmental covariates on their dynamics. Full descriptions of MAR models have been given elsewhere [6, 8, 10]; we summarize their structure here. MAR models are stochastic models that can be fit to species abundance time-series data to describe changes in abundance through time. MAR models simultaneously quantify community interactions and the effects of environmental covariates on abundance, while accounting for temporal autocorrelation in abundance data and population density dependence. In matrix form, MAR models are written as
where X t is a matrix of (log-transformed) community abundance vector at time t, A is a vector of constants representing species (or group) intrinsic growth rates; B is the community interaction matrix, with diagonal elements representing density-dependent self effects and off-diagonal elements representing effects between species (or groups), such as predation or competition; C is the matrix of environmental effects on species (or group) abundance; U t is a vector of environmental covariates at a chosen time lag n; and E t is a vector of process errors.
We fit MAR models to time series of plankton and exogenous driver data from Lake Aleknagik between 1963–2009. We grouped zooplankton into five categories: calanoid copepods, cyclopoid copepods, Daphnia, Bosmina, and Holopedium. Our model did not include primary producers because reliable historical chlorophyll-a concentrations do not exist. Zooplankton densities were aggregated to monthly averages, from June-September 1963–2009. Environmental and biological covariates included in the model were date of spring ice breakup, monthly water temperature (average 0–20 m), a seasonal term (month squared), predation by juvenile sockeye (estimated as adult sockeye escapement to Aleknagik in the previous year), and predation by threespine stickleback (estimated as their relative abundance in townet catches). Sockeye fry and threespine stickleback townet catches, sockeye escapement in the previous year, and ice breakup date were included in the model as annual estimates. The date of spring ice break up is expressed as the number of days after April 31 (i.e., May 1 = 1). Consistent with previously published studies (i.e. [7, 17, 18]), for all time series where data were missing, we used linearly interpolated values (requiring a total of 29 interpolations: interpolations for Holopedium on nine dates and interpolation for 0–20 m water temperatures on 20 dates, out of 301 total dates). If missing data were from June or September, we used the average of the values from the corresponding month in the year prior and the year after, assuming some degree of interannual temporal autocorrelation in the data. Zeros were replaced with a randomly generated number between zero and one half the lowest recorded value for that particular variable [per 12]. To characterize non-linear relationships more effectively [6], all data were ln(x + 1) transformed. To find the best model structure describing community interactions and environmental effects, i.e., which covariates should be included in the model, we started with a single covariate model and sequentially added covariates, evaluating the improvement in model fit of each additional covariate, using Akaike’s Information Criterion (AIC) to choose the most parsimonious model. We tested all covariate combinations and settled upon the model/models with the lowest AIC value. Each individual model was estimated using conditional least squares, and the best-fitting model for each structure was selected from 10,000 randomly-generated models (based on random E matrices; Hampton et al. (2006)). MAR modeling was performed in MATLAB (2007, The MathWorks), using the open-source program LAMBDA (Viscido and Holmes 2010; freely available from http://conserver.iugo-cafe.org/user/e2holmes/LAMBDA) with additional programming by the authors.
Future scenario simulations
We evaluated the influence of possible future climate on the presence and strength of species interactions by simulating community dynamics based on the best-fit model and alternative future climate drivers. We used MAR model parameters (A, B and C matrices) from the best-fit MAR model estimated as described above in combination with environmental scenarios to explore how changes in climate and predation might affect zooplankton community dynamics. We projected future zooplankton abundances using the MAR-estimated interactions in combination with future covariate scenarios. To simulate the effects of lake warming, we simulated 100-year temperature time-series, setting initial temperatures at the monthly long-term averages for lake surface (0–20 m), followed by a linear 2 °C increase over 100 years.
Daphnia are currently not a preferred zooplankton prey for planktivores in Lake Aleknagik (Schindler, unpublished data) presumably because they are found at such low densities throughout the summer, but we know that in other freshwater systems they are a critical component of the food web. Scheuerell et al. [34] documented that juvenile sockeye salmon do not preferentially feed on Daphnia until they achieve a density of at least 0.5 L−1, at which point the sockeye salmon show a non-linear switching response to feed almost exclusively on Daphnia. Our best-fit model did not detect a significant effect of sockeye fry on Daphnia (see Results) suggesting that Daphnia never achieve densities where they become the preferred prey for planktivorous fishes. However, if Daphnia production is enhanced under warmer conditions (see Results) it is possible that such a switching response in the planktivores may occur, thereby exerting more top-down pressure on Daphnia than they currently do.
To explore the potential impact of increased predation pressure on Daphnia that would result from a switching response by sockeye salmon we also simulated future plankton abundance under varying levels of predation intensity. We varied the C matrix coefficient corresponding to predation by sockeye salmon on Daphnia from 0 to -0.5, in increments of 0.05, under both constant climate conditions (i.e., using the long-term Lake Aleknagik average temperature for June-September) and the simulated climate warming described above. All other C matrix interaction coefficients remained the same as in the best-fit model. Simulation experiments were performed in R (R Core Team 2013).
Results
Long-term environmental patterns
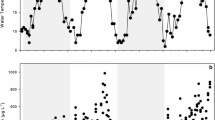
There has been a significant trend toward earlier spring ice breakup in Lake Aleknagik since 1963 (Fig. 2, p = 0.005), resulting in an average date of spring break up in 2009 that was 13 days earlier than in 1963. Positive trends in water temperature were significant for June, July, and August in the epilimnion (0–20 m stratum; Fig. 3, p = 0.002, 0.001, and 0.004, respectively). There was also an overall significant increasing trend in the annual average summer epilimnetic temperature (Fig. 3, p = 0.035). Average summer temperature increases in the top 20 m ranged from 0.03 to 0.05 °C/year. This translates into an overall temperature increase of 1.4–2.3 °C in the epilimnion between 1963 and 2009.
Important ecological interactions
The best model included significant effects of seasonality (month2), ice breakup date, epilimnetic (0–20 m) water temperature, and adult sockeye salmon escapement in the previous year on taxon-specific zooplankton abundance trends through time (Fig. 4, Table 1). Threespine stickleback abundance was not included in the most parsimonious model. All zooplankton taxa had positive autoregressive terms, implying that the density in time t had a positive influence on the density at time t +1. There were some significant competitive interaction terms: between Bosmina and Holopedium, between calanoid and cyclopoid copepods, and between Daphnia and Holopedium. Seasonality terms were positive for Bosmina and calanoid copepods and negative for cyclopoid copepods and Holopedium. There was no effect of season on Daphnia. The date of spring ice breakup had no effect on any taxa when included in a model with summer water temperatures. Temperature effects were positive for all cladocerans and negative for calanoid copepods. Negative effects of predation by juvenile sockeye salmon was only important for the copepods, the numerically dominant taxa in this plankton community.
Food web diagram showing major community interactions and the magnitude and direction of those interactions. Predation and competitive effects are noted in the upper portion of the figure. Autoregressive and environmental effects are shown in the bottom portion. Black arrows and numbers are positive interactions, blue arrows and numbers are negative
Effects of future climate change
Under scenarios of increasing lake surface temperature, taxa-specific densities varied annually, but only Daphnia exhibited a general trend toward increased abundances over the course of the simulation (Fig. 5). Bosmina and cyclopoid copepod densities remained unchanged, and there were general trends toward decreasing abundance of Holopedium and calanoid copepods (Fig. 5).
Results from 100 year simulations showing taxa-specific changes in zooplankton density with a 2 ° C increase in temperature. Year is on the x-axis, ln(density) is on the y-axis (note different scales for each taxon). The gray line is the simulation output. A linear trend line (dotted black line) and Lowess smoother with α = 0.1 [to approximate a 10-year moving average] (thick black line) were added to emphasize general trend directions
Simulated effects of future climate change and increased sockeye predation
Model simulations highlighted the importance of complex interactions between temperature and species interactions in determining community responses to warming. Calanoid and cyclopoid copepods showed no notable change in seasonal density with simulated increasing predation intensity (on Daphnia) alone, but cyclopoid densities were higher in the scenario that included both changes in predation intensity and climate warming (Fig. 6). Calanoid densities were lower when climate warming was included (Fig. 6). Seasonal Bosmina densities increased as predation intensity on Daphnia increased, and the increase was more pronounced when temperatures were warmer (Fig. 6). Holopedium densities increased as predation intensity on Daphnia increased, but warmer climate conditions actually moderated the rate of increase (Fig. 6). As expected, Daphnia densities declined as predation pressure upon them increased. In both climate scenarios (average and warmer conditions), the Daphnia population crashed at the highest predation intensities (Fig. 6). However, a warming climate enabled Daphnia to persist longer than if there were no positive effects of temperature on them.
Results from simulations showing taxa-specific changes in zooplankton density after 100 years across varying levels of predation intensity (on Daphnia) and a 2 ° C increase in temperature (grey circles), and changes in density with only varying levels of predation intensity on Daphnia (black circles). Predation intensity is on the x-axis, absolute density (ind/m2) is on the y-axis. Data are presented as absolute density rather than ln(density) to illustrate the actual functional response of each taxon, rather than linear trends. Density at each level of predation intensity is the average density from the final 10 years of the simulation
Discussion
Freshwater communities are structured by a combination of biotic and abiotic factors via both direct and indirect pathways. The zooplankton community in Lake Aleknagik appears to be primarily structured by bottom-up forcing. Spring ice breakup on Lake Aleknagik has shifted substantially earlier since 1963 (Fig. 2), depending on the type of trend model used (this paper and [26]). This is consistent with global increases in lake ice-free seasons due to either earlier spring ice break up, later freeze dates or a combination of both [35, 36]. The consequences of longer ice-free seasons and warmer water temperatures on freshwater crustacean zooplankton are apparent across the globe and are often seen as changes in density [37, 38], shifts in phenology [31, 32, 39,40,41,42], and alteration of reproductive strategies [42].
As the length of the summer growing season increased, we have also seen concomitant increases in epilimnetic water temperatures in all months of the summer since 1963 (Fig. 3). MAR model results suggest strong influences of climate, particularly water temperature, on zooplankton, with all cladocerans exhibiting positive responses and the calanoid copepods showing a negative response (there was no discernible effect of temperature on cyclopoid copepods) to warmer water temperatures. These results are consistent with similar analyses in other large lakes, where external temperature effects overshadowed intrinsic community interactions [17]. Like other poikilothermic organisms, the fundamental physiological processes of zooplankton are tightly linked to the temperature of their environment [43,44,45] and even slight increases in temperature can drive increases in density [46] and production [24, 47, 48]. Zooplankton, especially the cladocerans, and Daphnia in particular, have important trophic functions in freshwater food webs [49,50,51]. Given the strong influence of temperature on zooplankton, in the context of climate change, the trophic functions of zooplankton may be modified, and thus overall community or ecosystem functioning may be altered under warming conditions in high latitude lakes.
All communities are organized to some degree by biotic interactions, both between trophic levels (predation) and within trophic levels (competition). In communities with low species diversity, such as the zooplankton community of Lake Aleknagik, competition tends to act as a strong structuring agent, while predation tends to dominate in communities with many trophic levels [1, 52]. Our analysis of the Lake Aleknagik plankton community show that there are relatively few competitive interactions among species (Fig. 4). However, those competitive interactions may play an important role in future community structure. For example, temperature has a positive effect on the density of all three cladocerans, yet our simulations project decreases in Holopedium at higher temperatures. This is likely due to the strong combined competitive effect of both Daphnia and Bosmina on Holopedium (Table 1, Fig. 5).
One of our primary goals was to explore how complex community interactions may change under future potential environmental and predation scenarios. We focused on Daphnia, which our analyses suggested were a generally weak interactor in the Lake Aleknagik community, but are known to have key trophic functions in sockeye salmon rearing lakes [34]. Daphnia currently show competitive effects only on Holopedium, and were not sensitive to planktivore densities under recent environmental conditions. But due to the strong positive effect of temperature on Daphnia, it is possible that climate-induced warming may strengthen the competitive effects of Daphnia in this lake, or increase its densities to the point where it becomes a preferred prey for juvenile sockeye, much like it is in warmer and more productive lakes (e.g., Lake Washington [34]). Scheuerell et al. [34] showed that juvenile sockeye salmon showed no distinct preference for Daphnia over other members of the zooplankton community until their densities reached about 0.5 L−1 at which point sockeye salmon fry switched to feed nearly exclusively on Daphnia. The scenarios shown in Fig. 6 attempt to mimic such a switching response by exploring a range of values of the parameter describing the effects of sockeye salmon on Daphnia. We assume that the negligible interaction term fit from the historical data reflects the fact that Daphnia are currently a minor contributor to the zooplankton community. While MAR models do provide a formal way to project future community composition with changes in covariates, such simulations assume that the per capita interactions in the food web remain constant as defined during the period of time over which the model was fit. However, if further warming enhances Daphnia production [24], it is possible that the planktivore community will switch to feeding more specifically on Daphnia, thereby having effects on other members of the community in the future. Notably, Holopedium may see substantial increases in abundance as they are released from competition with Daphnia. Additionally, both cyclopoid and calanoid copepods have positive competitive interaction terms with Holopedium. Increasing predation pressure on Daphnia may result in a release of predation pressure on the copepods, thereby contributing to an even greater increase in abundance of Holopedium. Similarly, with increases in temperature plus increases in predation pressure on Daphnia, calanoid copepods decline in abundance, but not that much. This is most likely a result of the strong negative effect of temperature on calanoid abundance. But if an increase in Daphnia abundance due to climate warming results in a switching response of the predator from copepods to Daphnia, calanoids in particular may experience a release in predation pressure, and abundance may increase. Together, climate and predation interact to buffer calanoid copepods from sharp declines in abundance.
By changing a single interaction term (predation intensity on Daphnia) and simulating future plankton densities with and without climate warming, we demonstrate that climate has the ability to buffer negative effects of increased fish predation on zooplankton, but that the response varies by zooplankton taxa and depends on its sensitivity to both climate and predation, as well as interspecific community interactions. Given the strong response of Daphnia to simulated climate change and predation scenarios, this system may shift from being a copepod dominated system to one that is dominated by cladocerans, with Daphnia becoming increasingly more important as water temperatures increase. This is not unlikely, as Daphnia tend to be thermally constrained [53, 54], but are often the dominant zooplankton in many temperate lakes [34]. Thus, in the future, the zooplankton community of Lake Aleknagik may be similar to more southern lakes, like Lake Washington, in which the preferred prey of zooplanktivorous fish is often Daphnia. The strong response of the cladocerans, but especially Daphnia, to simulated climate change suggests that zooplankton communities may be able to support future increased growth rates or densities of freshwater fishes with ongoing climate warming.
Conclusions
As ecologists attempt to understand how ecological communities respond to climate change, there is concern that species interactions will conceal the response to climate. Analysis of the zooplankton community of Lake Aleknagik suggests that, while species interactions are important, the strongest structuring effects are actually the bottom-up temperature effects. Contrary to the common narrative that climate change will have detrimental effects on the majority of organisms in most locations around the globe, ongoing climate warming may actually increase the secondary productivity and the ecosystem services it supports (i.e. salmon fisheries) under a warmer climate in the near future.
References
Menge BA, Sutherland JP. Species-diversity gradients – Synthesis of roles of predation, competition, and temporal heterogeneity. Am Nat. 1976;110:351–69.
Arnott SE, Vanni MJ. Zooplankton assemblages in fishless bog lakes: Influence of biotic and abiotic factors. Ecology. 1993;74:2361–80.
Harley CDG. Abiotic stress and herbivory interact to set range limits across a two-dimensional stress gradient. Ecology. 2003;84:1477–88.
Paine RT. Food-webs – Linkage, interaction strength and community infrastructure – The 3rd Tansley Lecture. J Anim Ecol. 1980;49:667–85.
Wootton JT. Indirect effects and habitat use in an intertidal community – Interaction chains and interaction modifications. Am Nat. 1993;141:71–89.
Ives AR. Predicting the response of populations to environmental change. Ecology. 1995;76:926–41. doi:10.2307/1939357.
Hampton SE, Scheuerell MD, Schindler DE. Coalescence in the Lake Washington story: Interaction strengths in a planktonic food web. Limnol Oceanogr. 2006;51:2042–51. doi:10.4319/lo.2006.51.5.2042.
Holmes EE, Ward EJ, Wills K. MARSS: Multivariate Autoregressive State-space Models for Analyzing Time-series Data. R Journal. 2012;4:11–9.
Hampton SE, Holmes EE, Scheef LP, Scheuerell MD, Katz SL, Pendleton DE, Ward EJ. Quantifying effects of abiotic and biotic drivers on community dynamics with multivariate autoregressive (MAR) models. Ecology. 2013;94:2663–9. doi:10.1890/13-0996.1.
Ives AR, Dennis B, Cottingham KL, Carpenter SR. Estimating community stability and ecological interactions. Ecol Monogr. 2003;73:301–30. doi:10.1890/0012-9615(2003)073[0301:ECSAEI]2.0.CO;2.
Ives AR, Carpenter SR, Dennis B. Community interaction webs and zooplankton responses to planktivory manipulations. Ecology. 1999;80:1405–21. doi:10.1890/0012-9658(1997)080[1405:CIWAZR]2.0.CO;2.
Hampton SE, Schindler DE. Empirical evaluation of observation scale effects in community time series. Oikos. 2006;113:424–39. doi:10.1111/j.2006.0030-1299.14643.x.
Huber V, Gaedke U. The role of predation for seasonal variability patterns among phytoplankton and ciliates. Oikos. 2006;114:265–76. doi:10.1111/j.2006.0030-1299.14753.x.
Klug JL, Cottingham KL. Interactions among environmental drivers: Community responses to changing nutrients and dissolved organic carbon. Ecology. 2001;82:3390–403. doi:10.1890/0012-9658(2001)082[3390:IAEDCR]2.0.CO;2.
Francis TB, Wolkovich EM, Scheuerell MD, Katz SL, Holmes EE, Hampton SE. Shifting Regimes and Changing Interactions in the Lake Washington, U.S.A., Plankton Community from 1962–1994. PLoS ONE. 2014;9:e110363. doi:10.1371/journal.pone.0110363.
Griffiths JR, Hajdu S, Downing AS, Hjerne O, Larsson U, Winder M. Phytoplankton community interactions and environmental sensitivity in coastal and offshore habitats. Oikos. 2016;125:1134–43. doi:10.1111/oik.02405.
Hampton SE, Izmest’eva LR, Moore MV, Katz SL, Dennis B, Silow EA. Sixty years of environmental change in the world’s largest freshwater lake - Lake Baikal, Siberia. Glob Change Biol. 2008;14:1947–58. doi:10.1111/j.1365-2486.2008.01616.x.
Francis TB, Scheuerell MD, Brodeur RD, Levin PS, Ruzicka JJ, Tolimieri N, Peterson WT. Climate shifts the interaction web of a marine plankton community. Glob Change Biol. 2012;18:2498–508. doi:10.1111/j.1365-2486.2012.02702.x.
Lindegren M, Moellmann C, Nielsen A, Stenseth NC. Preventing the collapse of the Baltic cod stick through an ecosystem-based management approach. PNAS. 2009;106:14722–7. doi:10.1073/pnas.0906620106.
Mac Nally R, Thomson JR, Kimmerer WJ, Feyrer F, Newman KB, Sih A, Bennett WA, Brown L, Fleishman E, Culberson SD, Castillo G. Analysis of pelagic species decline in the upper San Francisco Estuary using multivariate autoregressive modeling (MAR). Ecol Appl. 2010;20:1417–30. doi:10.1890/09-1724.1.
Izmest’eva LR, Moore MV, Hampton SE, Ferwerda CJ, Gray DK, Woo KH, Pislegina HL, Krashchuk LS, Shimaraeva SV, Silow EA. Lake-wide physical and biological trends associated with warming in Lake Baikal. J Great Lakes Res. 2016;42:6–17. doi:10.1016/j.jglr.2015.11.006.
IPCC. Summary for policy makers. In: Pachauri RK, Meyer LA, editors. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC; 2014. p. 151.
Hartmann B, Wendler G. The significance of the 1976 Pacific climate shift in the climatology of Alaska. J Clim. 2005;18:4824–39. doi:10.1175/JCLI3532.1.
Carter JL, Schindler DE. Responses of zooplankton populations to four decades of climate warming in lakes of Southwestern Alaska. Ecosystems. 2012;15:1010–26. doi:10.1007/s10021-012-9560-0.
Westley P, Schindler DE, Quinn TP, Ruggerone GT, Hilborn R. Natural habitat change, commercial fishing, climate, and dispersal interact to restructure an Alaskan fish metacommunity. Oecologia. 2009;163:471–84. doi:10.1007/s00442-009-1534-3.
Schindler DE, Rogers DE, Scheuerell MD, Abrey CA. Effects of changing climate on zooplankton and juvenile sockeye salmon growth in southwestern Alaska. Ecology. 2005;86:198–209. doi:10.1890/03-0408.
Rich HB, Quinn TP, Scheuerell MD, Schindler DE. Climate and intraspecific competition control the growth and life history of juvenile sockeye salmon (Oncorhynchus nerka) in Iliamna Lake, Alaska. Can J Fish Aquat Sci. 2009;66:238–46. doi:10.1139/F08-210.
Eggers DM. Limnetic feeding behavior of juvenile sockeye salmon in Lake Washington and predator avoidance. Limnol Oceanog. 1978;23:1114–25.
Straile D, Geller W. The response of Daphnia to changes in trophic status and weather patterns: a case study from Lake Constance. ICES J Mar Sci. 1998;55:775–82. doi:10.1006/jmsc.1998.0397.
Straile D. Meteorological forcing of plankton dynamics in a large and deep continental European lake. Oecologia. 2000;122:44–50. doi:10.1007/PL00008834.
Winder M, Schindler DE. Climatic effects on the phenology of lake processes. Ecology. 2004;85:2011–106. doi:10.1111/j.1365-2486.2004.00849.x.
Winder M, Schindler DE. Climate change uncouples trophic interactions in an aquatic ecosystem. Glob Change Biol. 2004;10:1844–56. doi:10.1890/04-0151.
Stich HB, Brinker A. Oligotrophication outweighs effects of global warming in a large, deep, stratified lake ecosystem. Glob Change Biol. 2010;16:877–88. doi:10.1111/j.1365-2486.2009.02005.x.
Scheuerell JM, Schindler DE, Scheuerell MD, Fresh KL, Sibley TH, Litt AH, Shepherd JH. Temporal dynamics in foraging behavior of a pelagic predator. Can J Fish Aquat Sci. 2005;62:2494–501. doi:10.1139/f05-164.
Magnuson JJ, Robertson DM, Benson BJ, Wynne RH, Livingstone DM, Arai T, Assel RA, Barry RG, Card V, Kuusisto E, Granin NG, Prowse TD, Steward KM, Vuglinski VS. Historical trends in lake and river ice cover in the northern hemisphere. Science. 2000;289:1743–6. doi:10.1126/science.289.5485.1743.
Jensen OP, Benson BJ, Magnuson JJ, Card VM, Futter MN, Soranno PA, Stewart KM. Spatial analysis of ice phenology trend across the Laurentian Great Lakes region during a recent warming period. Limnol Oceanogr. 2007;52:2013–26. doi:10.4319/lo.2007.52.5.2013.
Straile D, Adrian R. The North Atlantic Oscillation and plankton dynamics in two European lakes – two variations on a general theme. Glob Change Biol. 2000;6:663–70. doi:10.1046/j.1365-2486.2000.00350.x.
Richardson AJ. In hot water: zooplankton and climate change. ICES J Mar Sci. 2008;65:279–95. doi:10.1093/icesjms/fsn0.
Gerten D, Adrian R. Climate-driven changes in spring plankton dynamics and the sensitivity of shallow polymictic lakes to the North Atlantic Oscillation. Limnol Oceanogr. 2000;45:1058–66.
Gerten D, Adrian R. Species-specific changes in the phenology and peak abundance of freshwater copepods in response to warm summers. Freshwater Biol. 2002;47:2163–73. doi:10.1046/j.1365-2427.2002.00970.x.
Adrian R, Wilhelm S, Gerten D. Life-history traits of lake plankton species may govern their phenological response to climate warming. Glob Change Biol. 2006;12:652–61. doi:10.1111/j.1365-2486.2006.01125.x.
Winder M, Schindler DE, Essington TE, Litt AH. Disrupted seasonal clockwork in the population dynamics of a freshwater copepod by climate warming. Limnol Oceanogr. 2009;54:2493–505. doi:10.4319/lo.2009.54.6_part_2.2493.
Bottrell HH, Duncan A, Gliwicz ZM, Grygierek E, Herzig A, Hillbricht-Ilkowska A, Kurasawa H, Larsson P, Weglenska T. Review of some problems in zooplankton production studies. Nor J Zool. 1976;24:419–56.
Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. Effects of size and temperature on developmental time. Nature. 2002;417:70–3. doi:10.1038/417070a.
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–51. doi:10.1126/science.1061967.
Visconti A, Manca M, De Bernardi R. Eutrophication-like response to climate warming: an analysis of Lago Maggiore (N. Italy) zooplankton in contrasting years. J Limnol. 2008;67:87–92. http://dx.doi.org/10.4081/jlimnol.2008.87.
Huntley ME, Lopez MDG. Temperature-dependent production of marine copepod: A global synthesis. Am Nat. 1992;140:201–42.
Shuter BJ, Ing KK. Factors affecting the production of zooplankton in lakes. Can J Fish Aquat Sci. 1997;54:359–77.
Carpenter SR, Cole JJ, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, Schindler DE. Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol Monogr. 2001;71:163–86. doi:10.1890/0012-9615(2001)071[0163:TCNALP]2.0.CO;2.
Sommer U, Sommer F, Santer B, Jamieson C, Boersma M, Becker C, Hansen T. Complementary impact of copepods and cladocerans on phytoplankton. Ecol Lett. 2001;4:545–50. doi:10.1046/j.1461-0248.2001.00263.x.
Sommer U, Sommer F. Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton. Oecologia. 2006;147:183–94. doi:10.1007/s00442-005-0320-0.
Kondoh M. Foraging adaptation and the relationship between food-web complexity and stability. Science. 2003;299:1388–91. doi:10.1126/science.1079154.
Gillooly JF. Effect of body size and temperature on generation time in zooplankton. J Plankton Res. 2000;22:241–51. doi:10.1093/plankt/22.2.241.
Gillooly JF, Dodson SI. Latitudinal patterns in the size distribution and seasonal dynamics of new world, freshwater cladocerans. Limnol Oceanogr. 2001;45:22–30. doi:10.4319/lo.2000.45.1.0022.
Acknowledgements
Bud Burgner and Don Rogers initiated and maintained the limnology program on the Wood River lakes until the late 1990s. Numerous students, faculty, and staff have contributed to data collection since 1963, notably Tom Quinn, Chris Boatright, and Ron Britton. Juvenile sockeye salmon data collection was approved under University of Washington IACUC permit #3142-01 and annual Alaska Department of Fish and Game collection permits.
Funding
The Alaska Salmon Program at the University of Washington has been supported by a variety of funding sources over its history, notably the Alaska salmon processing industry, the National Science Foundation, and the Gordon and Betty Moore Foundation. The funding bodies had no role in the design of the study, or collection, analysis, and interpretation of the data.
Availability of data and materials
Datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request. http://depts.washington.edu/aksalmon/data-requests/
Authors’ contributions
JC and DS conceived of the project idea. TF and JC ran the models and analyzed data, respectively. JC wrote the paper, with significant contributions from DS and TF. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable, no human or animal data used.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
Average Monthly Zooplankton Density. Average monthly zooplankton areal density (ind/m2) is shown for all taxa from 1963 to 2009. June densities are shown as the black line, July densities are blue, August are red, and September are green. The bottom left panel in the figure shows average monthly epilimnion (0–20 m) water temperatures in degrees Celsius over the same time period. There is substantial interannual variation for all taxa in all months (these figures include real data, without interpolations for missing values that were used in the MAR analysis). More information on patterns in zooplankton density/production rates in this community can be found in [24]. (PDF 566 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Carter, J.L., Schindler, D.E. & Francis, T.B. Effects of climate change on zooplankton community interactions in an Alaskan lake. Clim Chang Responses 4, 3 (2017). https://doi.org/10.1186/s40665-017-0031-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40665-017-0031-x