Abstract
Background
Urbanisation is a dominant geographical trend and an important component of global change, with unprecedented implications for socio-economic, cultural and environmental characteristics. However, green areas, including original fragments, can help to conserve native diversity, improving the functioning of these artificial systems in the long term. Urban areas can still provide habitats usable by wild birds, however the structural characteristics of the habitat formed by different types of green area differ, and therefore dissimilar bird diversities are to be expected. The object of this study was to characterise the α and β diversities of birds in different green areas and to analyse how diversity relates to ten variables that characterise the habitat.
Methods
We studied the green areas in the city of Temuco, southern Chile (Park, Square and Median strips of main streets), evaluating the variables: (a) surface area, (b) vegetation, (c) estimated human impact as the proportions of vegetation and bare soil by area, and the vehicle traffic. The bird assemblage structures were characterised by α (intra-environment) diversity and β diversity (between environments) and the statistical analysis identified the environmental variables related with the presence and abundance of birds. A statistical model was constructed to describe the contribution of the variables to bird diversity.
Results
We found significant differences between the diversity of bird species in the three types of green area. The β showed medium to high similarity between the different study units. There was a negative correlation with bare soil areas; the correlations with vehicle flow, plant structure and tree and shrub cover were not significant, meaning that these variables did not explain the variation in the richness of bird species between the green areas. However the surface area did explain this variation presenting a positive potential relation. There was also a high correlation with the origin (native) of shrub species.
Conclusions
The bird diversity varied significantly according to the type of urban green area. The environmental variables presenting significant correlations with bird diversity were: surface area, native species of shrub stratum, shrub cover, and bare soil area. The best multiple regression model showed that the three most important variables for bird diversity are the surface area of the green area, the cover of the shrub stratum and the presence of native shrub species.
Similar content being viewed by others
Background
The area covered by urban settlements is increasing rapidly, since the world’s urban population is expected to increase by 72% between 2000 and 2030 (Pickett et al. 2011). Urbanisation is a dominant geographical trend and an important component of global change, with unprecedented implications for socio-economic, cultural and environmental characteristics. This accelerated urbanisation process is a threat to biodiversity conservation (Clergeau et al. 2001; Pickett et al. 2011), and may cause alterations of ecological systems and malfunctioning of urban systems as they are presently structured (McDonnell et al. 1997). McNeely et al. (1990) define biological diversity as a concept that encompasses the variety of nature, including the number and frequency of ecosystems, species and genes; Halffter and Ezcurra (1992) recall that it is the result of the evolutionary process and Solbrig (1994) clarifies that it is not an entity, a resource, but a property or characteristic of nature.
Due to the fragmentation or complete elimination of the natural habitats over which cities are built or expanded, an urban system contains only a small surface area with fragments of original vegetation or even artificial green areas (Rees 1997). The result is a drastic change in fauna, with a reduction in the original abundance and diversity, as documented by various authors: birds (e.g., Blair and Launer 1997; Faggi 1998; Blair 1999; Cam et al. 2000); marsupials (e.g., Almeida et al. 2008); rodents (e.g., Cavia et al. 2009); bats (e.g., Oprea et al. 2009); amphibians (e.g., MacGregor-Fors et al. 2013); reptiles (e.g., Cossíos and Icochea 2006); and fish (e.g., Cunico et al. 2011).
Biotic homogenization is considered to be one of the most prominent forms of biotic impoverishment induced by current global changes. It is described as the process by which species invasions and extinctions increase the genetic, taxonomic or functional similarity of two or more biotas over a specified time interval (Olden 2006; Devictor et al. 2008; Knop 2016). It has been demonstrated that the homogenization of bird communities is strongly positively correlated to landscape disturbance and fragmentation (Devictor et al. 2008). Considering that cities tend to homogenize the physical environment, consequently, as they expand, biological homogenization increases because the urban-adaptable species become increasingly widespread and locally abundant (McKinney 2006). By that, the urban conservation should therefore focus on promoting preservation and restoration of local indigenous species and their habitats (McKinney 2006).
The rural bird communities compared with urban bird communities have lower average evolutionary distinctiveness (Morelli et al. 2016) and by other hand in the urbanized environments, changes in phylogenetic richness and evolutionary distinctiveness are less substantial in moderately urbanized environments that still preserve much of the original diversity (Sol et al. 2017). The urban environments are a factor of concern for maintaining diversity across the tree of life of birds, and the urbanization planning could help buffer extreme loss of phylogenetic diversity caused by this process (Morelli et al. 2016).
However, green areas, including original fragments, can help to conserve native diversity (e.g., flora, birds), improving the functioning of these artificial systems in the long term (e.g., Chace and Walsh 2006). This has been shown in various green areas such as urban gardens in Hamilton, New Zealand (Day 1995); private gardens, semi-public spaces associated with apartment buildings, public parks and courtyards of industrial buildings in Zurich, Lucerne and Lugano in Switzerland (Fontana et al. 2011); urban parks in Hong Kong (Zhoua et al. 2012); urban parks in Beijing (Morelli et al. 2017) and Santiago of Chile (Urquiza and Mella 2002). For further research in Latin America see Ortega-Álvarez and MacGregor-Fors (2011a). All these studies address bird diversity in disturbed and/or artificial habitats with species surviving from the original habitats. Interest in the urban ecology of birds is growing (Grimm et al. 2000, 2008; Garaffa et al. 2009); however, most research in Latin America has focused on species lists, which, although they provide valuable information, need to be complemented by other aspects such as demographic patterns, physiological and behavioural ecology, interactions between exotic and native species and adaptation processes and functional diversity (Ortega-Álvarez and MacGregor-Fors 2011a, b; MacGregor-Fors and Ortega-Álvarez 2013).
During the 19th century, the coastal mountain range in southern Chile between the Biobío River and Puerto Montt (approx. 36°‒41°S) was thickly forested. In the north–south lying central depression, the forest was not so dense; the woods which covered the gullies and hills alternated with cleared areas in the plains where the indigenous people practiced various forms of agriculture (Góngora 1862; de Vivar 1979; Camus 2002; Solari et al. 2011).
After the Spanish occupation, fragmentation of the native vegetation was intensified due to the expansion of farmland, particularly from the second half of the 19th century. The new structure of the countryside was dominated by continuous anthropic matrices, enclosing isolated fragments of secondary native forest (Gantz and Rau 1999; Rau and Gantz 2001). This process was accompanied and accelerated by strong urban expansion of the main cities of southern Chile. The impact of this urban expansion on bird communities appears to have been high, but knowledge is still at a rudimentary stage (Chace and Walsh 2006).
The objective of the present study was to analyse the bird diversity in green areas of the city of Temuco and to characterise the environments which the birds occupy, in order to answer the following questions. Which structural elements of green areas (e.g., vegetation strata) affect bird diversity and species richness the most? Is this avian diversity affected by the diversity and origin (native/exotic) of the vegetation? Is it affected by human disturbance (e.g., proportion of bare soil area, vehicle traffic adjacent to green areas)?
Methods
Study area
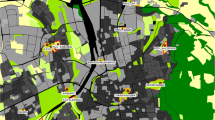
The study area comprised 10 green areas (study units) in the city of Temuco, located in the Araucanía Region, southern Chile (38°46ʹS, 72°38ʹW; 114 m asl), with a total urban area of 32.54 km2. In 2007 the population was 287,000 with a density of 4 m2 of green areas, maintained by the city council, per inhabitant of the comuna (district) of Temuco (Ministerio del Interior 2009). Urban areas can still provide habitats usable by wild birds; however, the characteristics of the habitat formed by different types of green area differ, and therefore dissimilar bird diversities are to be expected. The study units were: (a) five town squares (Godoy, Recabarren, Pinto, Dreves and Schmidt). Town squares are open, square shaped spaces for public use, connected by streets and used principally for leisure and pedestrian traffic; they are civic spaces for exchange relationships within a neighbourhood determined by sociability and movement to maximize human contacts (Miyasako 2009); in Chile, these spaces contain trees and gardens; (b) two median strips of main streets (Balmaceda and Prieto Norte). Median strips are green areas that exist in the central axis of a great avenue. In this context we refer to median strips set along these two major streets (approx. 30 and 20 m wide respectively), which are covered with herbaceous vegetation, shrubs and trees; (c) three urban parks (Germán Becker, Los Pinos and Campus Norte of Universidad Católica de Temuco) (Fig. 1).
The climate of the study area is wet-temperate. The average annual precipitation is 1400 mm, distributed throughout the year, with greater intensity in the winter and one or two dry months in summer; the average annual temperature is 12 °C (Di Castri and Hajek 1976). The local vegetation formation is southern deciduous forest (Gajardo 1994) and the urban flora of Temuco, found principally in the green areas, consists mainly of exotic species (Hauenstein and Leiva 1987; Hauenstein and Latsague 1988; Hauenstein et al. 1988, 1989). The greatest distance between study units is 4990 m and the least is 226 m.
Characterization of the study units
The study was carried out over one year, from southern autumn 2007 to southern summer 2008. The following variables were assessed in all the study units (town squares, median strips of main streets, parks) for: (a) surface area measured in ha; (b) vegetation measured as species richness of herbs, plants, and trees; number of individuals of each species in each stratum (herbaceous, shrub and tree, perennial and deciduous); the plant cover was estimated by a floristic inventory including the number of each species. In the squares and median strips of main streets the samplings included all the surface of squares (0.9‒1.1 ha) and median strips of main streets (2‒3.7 ha) and the parks were surveyed in square subplots of 20 m × 20 m each, in a variable number according to the surface of each park (5‒17 ha). The species were included in an inventory with scientific name and phytogeographic origin (sensu Hoffmann 1991, 1999; Tykac 1993; Hessayon 1999). We also recorded the proportion (%) of cover to the total surface area of each study unit; in each plot all vascular plant species present were identified and for each was estimated its relative abundance (sensu Mueller-Dombois and Ellenberg 1974); (c) estimated human impact as the proportions of vegetation and bare soil by area (soil without vegetation or covered by cement), and the vehicle traffic flow on the roads adjacent to each study unit, estimated in number of vehicles per minute measured at the same hour in the same place and reduced to an annual average (modified from Leveau and Leveau 2006), the counting of the vehicles was done in the morning when the highest vehicle flow occurs; this variable was not considered in parks as there is no vehicle traffic. The bare soil area was estimated as a proportion of the total area of each study unit and was considered to be a human impact variable, not a type of habitat, as its characteristics do not make it an appropriate habitat for birds (see Faggi and Perepelizin 2006).
Census methods
In town squares we used the point count method proposed by Bibby et al. (1993); counting points were located 25 m apart and the counting period at each point was 10 min. The census uses sighting/listening points with a 25 m radius according to Hutto et al. (1986), Bibby et al. (1993) and Willson et al. (1994). In median strips the method was adapted (sensu Leveau and Leveau 2004) to the variable dimensions of the median strips of main streets (linear areas), for periods of 5‒10 min. Sampling in parks covered all the different habitats in each park (Faggi and Perepelizin 2006). The census points were independent since two observers participated simultaneously, one of them with the objective of avoiding double counting when identifying the birds that could move from one plot to another. The sampling intensity was determined, by means of previous censuses, with the rarefaction method (accumulation curves) (sensu Willson et al. 1994) and the whole surface area of each unit was sampled. Water birds were excluded, because only one study unit contained wetland habitat (Campus Norte, a small lagoon with six species).
A total of 220 counts were carried out: 112 in town squares and median strips of main streets, and 108 in parks. All the study sites were sampled in each season of the year, with four replicates (4 days) with a total sampling effort of 160 censuses. The frequency was 9 days/season (autumn, winter, spring, summer) for parks and 4 days/season for town squares and median strips. In each case counting was carried out between 7:00 am and 12:00 noon, when birds presented their greatest feeding and social activity. The total period covered a complete annual biological cycle from southern autumn 2007 to southern summer 2008. The total number of counts was 16 in town squares and median strips of main streets (4 per season) and 36 in parks (9 per season).
The censuses were taken by two observers trained in visual and aural bird identification (Verner and Milne 1989), in order to capture all the birds in the census with no counting errors. Nomenclature followed Marín (2004). Both the native and exotic bird species identified were included in the records.
Analysis of bird assemblage structures
The bird assemblage structures in each of the study units were characterised by: (a) The α (intra-environment) diversity measured as species richness (S) and Shannon–Wiener’s diversity index, which quantifies the total diversity of a sample influenced by two basic components, richness and evenness. The formula for this function is: Hʹ = − Σ (pi × log2 pi), where pi is the proportion of the total number of individuals in the sample corresponding to the species. The values ranged between zero, when there was only one species, and the maximum (\(H_{\max }^{\prime }\)) corresponding to log2 S. In addition, the Pielou (J) index was calculated according to the equation: J = Hʹ/\(H_{\max }^{\prime }\). This index describes species evenness of a community. Hence it measures the proportion of the diversity observed (Hʹ) in relation to the maximum diversity expected (\(H_{\max }^{\prime }\)). Its values fluctuate between 0 (minimum heterogeneity) and 1 (maximum heterogeneity, i.e. the species are equally abundant) (Magurran 1998; Magurran and McGill 2011; Maurer and McGill 2011). To test the null hypothesis that the Hʹ diversities of the four environments were equal, Hutcheson’s procedure (1970) described in Zar (2010) was followed. This consists of a t test to calculate the weighted diversity index (Hp = (NlogN) − (Σ pi log pi)/N), including the variance calculation for each environment according to \(S_{{H^{\prime } }}^{2}\) = [Σ (pi log)2pi − (Σ pi log pi)2/N]/N2. We processed this test in a programme created by the authors in an Excel spread sheet; (b) The β diversity (between environments) was represented by the differentiation between the study units using a dissimilarity tree diagram based on the Bray–Curtis index (1957) and the UPGMA unweighted pair group method with arithmetic mean (Sokal and Rohlf 1995), using the software Past 1.68 (Hammer et al. 2001). The level of significance (p = 0.05) of the dendrogram results was determined by calculating the 95% percentile of similarity pseudoranges obtained by the bootstrapping technique with 10,000 iterations (Manly 1997). Various measures of β diversity exist (see Koleff et al. 2003); a similarity tree diagram was used in this work because it allows comparison with other similar research in Chile.
Statistical analysis
Non-metric multidimensional scaling (nMDS) was used to distinguish similar groups of study units (town squares, median strips of main streets, parks) considering structural variables (e.g., vegetation covered by stratum), vegetation characteristics (e.g., richness and origin) and human impact (e.g., bare soil area, noise pollution from vehicle traffic). In addition a hierarchical agglomerative clustering analysis was performed applying a square root transformation to the original data and using Bray–Curtis as an index of similarity. To objectively identify the groupings, the SIMPROF test was carried out (Clarke et al. 2014).
It was established if the relative abundances of the birds in a study unit, as a whole, differ among sites (ANOSIM). Analysis of the species which contributed to the differences in bird composition between study units was done by similarity percentage analysis (SIMPER). To identify the environmental variables (e.g., structural, vegetational and anthropic impact) which explained the multivariate community pattern of birds in the study units, a BEST procedure (Biota and/or Environment matching) was carried out in the Primer 6.0 software, with forward stepwise Spearman Rank correlation (Clarke and Gorley 2006). Finally, a statistical model was constructed to describe the contribution of the variables to bird diversity using step by step Multiple Regression as per Zar (2010). Normality was assessed for each variable by graphical analysis and the Chi square test; the homogeneity of variances was evaluated by Bartlett’s Test. The statistical programmes used were XLSTAT version 4.03, Statgraphics plus 5.1 and PRIMER version 6.
Results
Characterisation of study units
The largest study units were the parks (9.5‒17 ha), and the smallest were the squares (≈ 1 ha) (Table 1). The highest richness of tree species was found in two of the parks (Becker and Campus Norte), and the lowest in the Los Pinos Park and Recabarren Square; for the shrub stratum, the highest values were recorded in two town squares and the lowest in a park and a square. The tree cover in all the study units was high (≥ 30%), while the shrub cover was low (< 10%) and the herbaceous stratum was variable (between 0 and 50%) (Table 1). There was dissimilarity in the composition of the flora in the study units (≥ 60%), in both the tree and shrub strata; the dissimilarity was the greatest in the shrub stratum (Fig. 2).
In terms of human disturbance, the greatest proportion of soil without vegetation (bare soil area) was in the town squares and median strips of main streets (40%‒55%), except in Recabarren Square; the lowest proportion was in the parks (< 10%). The highest rate of vehicle flow was recorded in the median strips of the main streets (> 223 vehicles/min). In the squares the flow ranged from 27 to 76 vehicles/min, and in the parks the vehicle flow was not recorded because none exists (Table 1). In terms of phyto-geographical origin, the majority of the study units presented a high percentage of exotic species (≤ 70%), in both trees and shrubs. The unit with the highest percentage of native species was Campus Norte (82%). On the other hand Los Pinos park was composed of a single, exotic species (Pinus radiata D. Don) (Fig. 3).
Bird abundance and diversity
The number of individuals recorded was 5282, from 30 bird species belonging to 7 orders and 18 families. The abundance, the species richness and Shannon’s index (Hʹ), and the taxonomic representation for the study units are shown in Table 2. The highest number of individuals was recorded in two parks (Campus Norte and Becker > 668), and the lowest in a square (Recabarren < 132). In the other study units, the numbers ranged from 343 to 692 individuals. The greatest diversity of birds as richness of species (S) (24) was recorded in a park (Campus Norte) and the lowest (≤ 9) in two squares (Godoy and Pinto) (Table 2). The highest values for the Shannon index (Hʹ) were recorded in the Campus Norte and Los Pinos parks, and the lowest in two squares (Pinto and Schmidt). For the homogeneity of species distribution, the highest evenness index (J) was found in Los Pinos park and the lowest in two squares (Schmidt and Godoy). The unit which presented the greatest taxonomic representation in the number of orders and families was a park (Campus Norte) while the lowest representation was in a square (Pinto).
In the parks, 91.2% of the individuals were native (belonging to 35 species). In town squares and median strips only 38.3% of the individuals were native (belonging to 22 species), while the exotic species Passer domesticus accounted for 60.3% of all individuals.
The β diversity presents medium to high similarity between the different study units (> 40% in the 10 green areas). There were not clear associations between types of units. Only two clusters were significant (bootstrap 100%), one that groups the Parks Campus Norte and Becker with 50% of similarity and other group that includes all the other areas with > 40% of similarity. The most similar areas were the squares Godoy, Pinto and Schmidt with > 73% of similarity and 16 shared species (Fig. 4).
Analysis of habitat variables
Multidimensional scaling analysis showed that parks formed one group, and town squares and median strips formed the other group (Fig. 5). There were significant global differences (p = 0.014), corroborated by the ANOSIM test. Pair-wise comparisons indicated significant differences between parks and squares (p = 0.018) and between parks and median strips of main streets (p = 0.010), but not between squares and median strips (p = 0.66). Parks and squares presented a dissimilarity of 65.33%; the principal species contributing to the difference were P. domesticus (31.57%) and Milvago chimango (9.64%). The difference between parks and median strips of main streets was 53%, resulting mainly from the abundance of P. domesticus, M. chimango and Sicalis luteola. P. domesticus and M. chimango were the most abundant in median strips while Vanellus chilensis was the most abundant in parks.
Dissimilarities represented by Non metric multidimensional scaling (nMDS) (Transform = Square root; Resemblance = Bray–Curtis similarity; 2-d: Stress: 0.07) between the types of green area in terms of species richness, area (in ha), native origin of the shrub stratum, tree cover and bare soil area in 10 green areas in Temuco, southern Chile, 2007–2008
According to results of the BEST routine, the environmental variables (surface area, native species of shrub stratum, shrub cover and bare soil area) explain the 84% of the variability in bird diversity. After a step by step multiple regression analysis was carried out with the environmental variables, the best of all models was ‘Bird diversity = 12.6636 + 0.696962 × Surface area of study unit − 0.700773 × Shrub Cover + 0.239708 × Native species of shrub stratum’ with r2 = 0.935, p-value of 0.05 and Mallows’ Cp of 0.27. The variables selected in this model coincided with those previously identified by BEST routine.
Discussion
In our study we found out that the three most important variables for bird diversity were the surface area, the cover of the shrub stratum and the presence of native species of shrub. So the structural elements of green areas affect bird diversity. This diversity is affected by some human disturbance like the bare soil areas, but not by vehicle flow, plant structure and tree and shrub cover.
Willson and Armesto (2003) stated that the consequences of fragmentation for bird species depend on their habitat requirements and their home range, and that larger areas, such as parks, are more likely to be colonised by rare or randomly distributed species. The surface area would therefore explain the greater diversity (species richness) in the parks as compared to squares and median strips of main streets; however, the diversity was also correlated with the number of tree species in the squares, the presence of shrub species of native origin and the absence of bare soil areas. It seems that native shrubs present in the study area offer more food (e.g., Ugni molinae, Berberis buxifolia, Berberis darwini, Fuchsia magellanica).
In squares and median strips of main streets, the majority of bird species were exotic (60%), which was rather less than the percentage documented in some European cities (80%) (e.g., Blair and Launer 1997; Chace and Walsh 2006). This is explained by the lack of natural habitat (high proportion of bare soil areas) and intolerance to human disturbance (Vejrup 2004), for this reason the exotic species Passer domesticus is a bird associated with anthropized environments around the world (De Laet and Summers-Smith 2007). In this study a significant linear relation was demonstrated between bird diversity (species richness) on the one hand, and the surface area of the units, the shrub cover and the presence of native species on the other. The lower diversity of birds in squares and median strips of main streets compared to their diversity in parks may be explained by the smaller surface area and isolation in a hostile matrix (Fahrig 2003). In this type of green area the surface areas are small (< 4 ha) and structurally simple, with geometric architecture, rectangular in the squares and linear in the median strips of main streets. In addition, their location in the city centre results in the predominance of tall buildings all around, narrow streets with high vehicle flows, low or poor plant cover and large bare soil areas, similar to the situation described in other Southern Hemisphere cities with low diversity (e.g., Garitano-Zavala and Gismondi 2003; Leveau and Leveau 2004, 2006; Faggi and Perepelizin 2006; Pauchard et al. 2006). Clergeau et al. (2001) and Jokimäki and Kaisanlahti-Jokimäki (2003) reported that cities offer a habitat for birds independent of the phyto-geographical origin of the plants (native/exotic) because plants are a scarce resource. In this study this was true for the origin of tree species, but the native origin of shrub species was positively correlated with bird diversity.
With respect to disturbance caused by vehicle traffic, Chace and Walsh (2006) reported that it causes isolation of populations, alteration of acoustic communication patterns, and direct mortalities due to collisions with vehicles, reducing the recreation value of green areas as well as their value as fauna corridors (Nilsson and Randrup 1997). In this study, however, we found no significant correlation between vehicle flow and bird species richness. Although the bare soil area variable presented a significant negative correlation with bird species richness in town squares and median strips of main streets, it was not considered in the model as it does not occur in the three parks.
This is a very interesting case of urban ecosystem, because of the environmental characteristics of the city (e.g., high air pollution, low surface of green areas, vegetation ecotone between sclerophyll and temperate rainforest), and the proximity to protected areas (Ñielol, Rucamanque) (Romero et al. 2001). The landscaping of the green areas in Temuco is European in style, characterised by exotic tree and shrub species and little native flora. These mosaics of small-scale habitats contain a high diversity of vegetation but are very small in area, with the character of plant collections. Anguita (2003) proposed that many of these exotic plant species (shrubs and trees) have little value for native bird species especially because they have a low food supply. The preference of town planners and urban landscapers for exotic species does not favour the creation of habitats suitable for birds, which is why many birds only visit these green areas but do not reside or nest there. Furthermore the vegetation in green areas is subjected to regular intervention (e.g., pruning, fumigation and removal of dead plants), displacing bird species which require intact plant cover, dead stumps, plant litter, or a quiet habitat (Lim and Sodhi 2004). Finally, urbanisation tends to select omnivorous or grain-eating species, and those which nest in cavities, and drives the progressive loss of insectivorous and nectarivorous species dependent on structurally diverse and/or native vegetation for both shelter and food resources, so this type of habitat increases avian biomass but reduces diversity (White et al. 2005).
Greater connectivity increases the area of habitat that birds can occupy, resulting in greater species richness, while a larger surface area of green areas would allow greater abundance due to an increase in the habitat available to the species already present in a fragment (Shanahan et al. 2012). A combined focus on improving the connectivity and the habitat in terms of life forms, diversity and connectivity (e.g., higher number of tree species, greater proportion of native shrub species, soil with plant cover, plants which provide food) would lead to an increase in the richness of bird species in green areas. For future research, it would be interesting to study their relationships with nearby protected areas, and the habitat requirements for the different bird species and their relationships with different vegetation strata and plant functional groups.
Ecological research has moved into the cities, and the challenge is to put knowledge into practice, with regulatory policies based on incentives which influence the design of green infrastructure to really conserve and increase biodiversity (Hostetler et al. 2011; Steiner 2011). For all this it is recommended in the planning and management of the green areas of cities in the south of Chile, to include native vegetation, especially increasing its cover (in park, square and median strips of main streets) and, as much as possible, increasing its surfaces. To increase the avian diversity we propose, for park and square, increasing the coverage of shrubs and trees of different heights and as far as possible to decrease the bare soil. For median strips it is proposed to increase the coverage of shrubs and medium height trees.
Conclusions
The bird diversity varied significantly according to the type of urban green area. Greater diversity, expressed as species richness and evenness, was observed in parks than in town squares and median strips of main streets. The environmental variables presenting significant correlations with bird diversity were: surface area, native species of shrub stratum, shrub cover, and bare soil area. The BEST multiple regression model showed that the three most important variables for bird diversity are the surface area of the green area, the cover of the shrub stratum and the presence of native shrub species. All these variables should be considered by urban planners in the construction or modification of urban green areas, in order to avoid biotic homogenization.
References
Almeida AJ, Torquestti CG, Talamoni SA. Use of space by neotropical marsupial Didelphis albiventris (Didelphimorphia: Didelphidae) in a urban forest fragment. Rev Bras Zool. 2008;25:214–9.
Anguita R. Animales en la gran ciudad (Animals in the big city). Ambienta. 2003;20:32–7.
Bibby CJ, Burges N, Hill V. Bird census techniques. British trust for ornithology. Royal society for the protection of birds. San Diego: Academic Press; 1993.
Blair RB, Launer AE. Butterfly diversity and human land use: species assemblages along an urban gradient. Biol Conserv. 1997;80:113–25.
Blair RB. Birds and butterflies along an urban gradient: surrogate taxa for assessing biodiversity? Ecol Appl. 1999;9:164–70.
Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 1957;27:325–49.
Cam E, Nichols JD, Sauer JR, Hines JE, Flather CH. Estimation of relative species richness for assessing the degree of completeness of ecological communities: avian communities and urbanization in the Mid-Atlantic states. Ecol Appl. 2000;10:1196–210.
Camus P. Bosques y tierras despejadas en el período de la conquista de Chile. In: Retamales J, editor. Forest and land cleared in the period of the conquest of Chile. Estudios coloniales II. Santiago de Chile: Editorial Biblioteca Americana; 2002.
Cavia R, Cueto GR, Suarez OV. Changes in rodent communities according to the landscape structure in an urban ecosystem. Landsc Urban Plan. 2009;90:11–9.
Chace J, Walsh J. Urban effects on native avifauna: a review. Landsc Urban Plan. 2006;74:46–69.
Clarke K, Gorley R. Plymouth routines in multivariate ecological research. London: Primer-E. Plymouth Marine Laboratory; 2006.
Clarke K, Gorley R, Somerfield P, Warwick R. Change in marine communities: an approach to statistical analysis and interpretation. Devon. 3rd ed. Ivybridge: Primer-E Ltd Lutton; 2014.
Clergeau P, Jokimäki J, Savard JPL. Are urban bird communities influenced by the bird diversity of adjacent landscapes? J Appl Ecol. 2001;38:1122–34.
Cossíos ED, Icochea J. Nuevos registros para el gecko de Lima, Phyllodactylus sentosus (Reptilia, Geckonidae). Ecol Appl. 2006;5:182–4.
Cunico AM, Allan JD, Agostinho AA. Functional convergence of fish assemblages in urban streams of Brazil and the United States. Ecol Indic. 2011;1z:1354–9.
Day TD. Bird species composition and abundance in relation to native plants in urban gardens, Hamilton, New Zealand. Notornis. 1995;42:172–86.
Devictor V, Julliard R, Clavel J, Jiguet F, Lee A, Couvet D. Functional biotic homogenization of bird communities in disturbed landscapes. Glob Ecol Biogeogr. 2008;17:252–61.
Di Castri F, Hajek F. Bioclimatología de Chile. Bioclimatology of Chile. Santiago de Chile: Publicaciones Vicerrectoría Académica; 1976.
Faggi A, Perepelizin P. Riqueza de aves a lo largo de un gradiente de urbanización en la ciudad de Buenos Aires. Rev Mus Argentino Cienc Nat. 2006;8:289–97.
Faggi AM. The influence of NGOs in preserving green places in Buenos Aires City. In: Breuste JH, Uhlmann O, editors. Urban ecology. Berlin: Springer; 1998.
Fahrig L. Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst. 2003;34:487–515.
Fontana S, Sattler T, Bontadina F, Moretti M. How to manage the urban green to improve bird diversity and community Structure. Landsc Urban Plan. 2011;101:278–85.
Gajardo R. La vegetación natural de Chile. Clasificación y distribución geográfica. [The natural vegetation of Chile. Classification and geographical distribution]. Santiago de Chile: Editorial Universitaria; 1994.
Gantz A, Rau J. Relación entre el tamaño mínimo de fragmentos boscosos y su riqueza de especies de aves en el sur de Chile. An Museo Hist Nat Valp. 1999;24:85–90.
Garaffa P, Filloy J, Bellocq MI. Bird community responses along urban-rural gradients: does the size of the urbanized areas matter? Landsc Urban Plan. 2009;90:33–41.
Garitano-Zavala A, Gismondi P. Variación de la riqueza y diversidad de la ornitofauna en áreas verdes urbanas de las ciudades de La Paz y El Alto, Bolivia. Ecol Boliv. 2003;38:65–78.
Góngora A. Historia de Chile desde su descubrimiento hasta el año 1575. History of Chile since its discovery until 1575. Colección de Historiadores de Chile. Tomo 11, Santiago de Chile: Imprenta del Ferrocarril; 1862.
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. Global change and the ecology of cities. Science. 2008;319:756–60.
Grimm NB, Grove JM, Pickett STA, Redman CL. Integrated approaches to long-term studies of urban ecological systems. Bioscience. 2000;50:571–84.
Halffter G, Ezcurra E. ¿Qué es la biodiversidad? In: Halffter G, editor. La diversidad biológica de Iberoamérica I, Volumen Especial. Ciudad de México: Acta Zoológica Mexicana; 1992.
Hammer O, Harper DA, Ryan PD. PAST: palaeontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9.
Hauenstein E, Latsague M. Evaluación florística y sinecológica del Monumento Natural Cerro Ñielol (IX Región, Chile). Bol Mus Reg Arauc. 1988;3:7–32.
Hauenstein E, Leiva L, González M. Los árboles y arbustos de las plazas de Temuco: II Plazas Recabarren y Teodoro Smith. Árb Nues Ami. 1988;1:43–6.
Hauenstein E, Leiva L, González M. Los árboles y arbustos de las plazas de Temuco: III Plazas Dagoberto Godoy y Dreves. Árb Nues Ami. 1989;1:49–53.
Hauenstein E, Leiva L. Los árboles y arbustos de las plazas de Temuco: I Plaza Aníbal Pinto. Árb Nues Ami. 1987;3:18–20.
Hessayon DG. Árboles y arbustos de jardín: manual de cultivo y conservación. Madrid: Editorial Blume; 1999.
Hoffmann A. El árbol urbano en Chile. 3rd ed. Santiago de Chile: Ediciones Fundación Claudio Gay; 1999.
Hoffmann A. Flora silvestre de Chile zona araucana. 2nd ed. Santiago de Chile: Ediciones Fundación Claudio Gay; 1991.
Hostetler M, Allen W, Meurk C. Conserving urban biodiversity? Creating green infrastructure is only the first step. Landsc Urban Plan. 2011;100:369–71.
Hutcheson K. A test for comparing diversities based on Shannon formula. J Theor Biol. 1970;29:151–4.
Hutto RL, Pletschet M, Hendricks P. A fixed-radius point count method for nonbreeding & breeding season use. Auk. 1986;103:593–602.
Jokimäki J, Kaisanlahti-Jokimäki M. Spatial similarity of urban bird communities: a multiscale approach. J Biogeogr. 2003;30:1183–93.
Knop E. Biotic homogenization of three insect groups due to urbanization. Glob Change Biol. 2016;22:228–36.
Koleff P, Gaston KJ, Lennon JJ. Measuring beta diversity for presence–absence data. J Anim Ecol. 2003;72:367–82.
De Laet J, Summers-Smith JD. The status of the urban house sparrow Passer domesticus in north-western Europe: a review. J Ornithol. 2007;148(Suppl 2):S275–8.
Leveau CM, Leveau LM. Ensambles de aves en calles arboladas de tres ciudades costeras del sudeste de la Provincia de Buenos Aires, Argentina. El Hornero. 2006;21:25–30.
Leveau M, Leveau C. Comunidades de aves en un gradiente urbano de la ciudad de Mar del Plata, Argentina. El Hornero. 2004;19:13–21.
Lim H, Sodhi N. Responses of avian guilds to urbanisation in a tropical city. Landsc Urban Plan. 2004;66:199–215.
MacGregor-Fors I, Hernández Ordoñez O, Ortega-Álvarez R. Urban croaking: diversity and distribution of anurans in a neotropical city. Urban Ecosyst. 2013;16:389–96.
MacGregor-Fors I, Ortega-Álvarez R. Ecología Urbana: Experiencias en América Latina. 2013. www1.inecol.edu.mx/libro_ecologia_urbana.
Magurran A. Ecological diversity and its measurement. Princeton: Princeton University Press; 1998.
Magurran AE, McGill BJ. Biological diversity frontiers in measurement and assessment. Princeton: Oxford University Press; 2011.
Manly BF. Randomization, bootstrap and MonteCarlo methods in biology. Boca Raton: Chapman and Hall; 1997.
Marín M. Lista comentada de las aves de Chile [Annotated list of the birds of Chile]. Santiago de Chile: Lynk Ediciones; 2004.
Maurer BA, McGill BJ. Measurement of species diversity. In: Magurran AE, McGill BJ, editors. Frontiers in measurement and assessment. Oxford: Oxford University Press; 2011.
McDonnell MJ, Pickett STA, Groffman P, Bohlen P, Pouyat RV, Zipperer WC, Parmelee RW, Carreiro MM, Medley KY. Ecosystem processes along an urban-to-rural gradient. Urban Ecosyst. 1997;1:21–36.
McKinney ML. Urbanization as a major cause of biotic homogenization. Biol Conserv. 2006;127:247–60.
McNeely JA, Miller KR, Reid WV, Mittermeier RA, Werner TB. Conserving the world’s biological diversity. Gland: International Union for the Conservation of Nature and Natural Resources, World Resources Institute, Conservation International, WWF US and World Banks; 1990.
Ministerio del Interior. Indicador: Áreas verdes con mantenimiento por habitante. Subsecretaria de Desarrollo Regional y Administrativo (Subdere). Sistema Nacional de Indicadores Municipales (Sinim). 2009. http://www.observatoriourbano.cl/indurb/indicadores.asp?id_user=&id_indicador=10&idComCiu=1. Accessed 22 Jan 2016.
Miyasako E. Las áreas verdes en el contexto urbano de la ciudad de México. Thesis Universidad de Alicante, Spain. 2009.
Morelli F, Benedetti Y, Ibáñez-Álamo JD, Jokimaki J, Mänd R, Tryjanowski P, Møller AP. Evidence of evolutionary homogenization of bird communities in urban environments across Europe. Global Ecol Biogeogr. 2016;25:1284–93.
Morelli F, Benedetti Y, Su T, Zhou B, Moravec D, Šímová P, Liang W. Taxonomic diversity, functional diversity and evolutionary uniqueness in bird communities of Beijing’s urban parks: effects of land use and vegetation structure. Urban For Urban Green. 2017;23:84–92.
Mueller-Dombois D, Ellenberg H. Aims and methods of vegetation ecology. New York: Wiley; 1974.
Nilsson K, Randrup TB. Silvicultura urbana y periurbana [Urban and periurban forestry]. In: CEPAL, editor. Actividades forestales urbanas y periurbanas. Ciudad de México: Editorial CEPAL; 1997. p. 85–104.
Olden JD. Biotic homogenization: a new research agenda for conservation biogeography. J Biogeogr. 2006;33:2027–39.
Oprea M, Mendes P, Vieira TB, Ditchfield AD. Do wooded streets provide connectivity for bats in an urban landscape? Biodivers Conserv. 2009;18:2361–71.
Ortega-Álvarez R, MacGregor-Fors I. Dusting off the file: a review of knowledge on urban ornithology in Latin America. Landsc Urban Plan. 2011a;101:1–10.
Ortega-Álvarez R, MacGregor-Fors I. Spreading the word: the ecology of urban birds outside the United States, Canada, and Western Europe. The Auk. 2011b;128:415–8.
Pauchard A, Aguayo M, Peña E, Urrutia R. Multiple effects of urbanization on the biodiversity of developing countries: the case of a fast-growing metropolitan area (Concepción, Chile). Biol Conserv. 2006;127:272–81.
Pickett STA, Cadenasso ML, Grove JM, Boone CG, Groffman PM, Irwin E, Kaushal SS, Marshall V, McGrath B, Nilon CH, Pouyat RV, Szlavecz K, Troy A, Warren P. Urban ecological systems: Scientific foundations and a decade of progress. J Environ Manag. 2011;92:331–62.
Rau J, Gantz A. Fragmentación del bosque nativo del sur de Chile: efectos del área y la forma sobre la biodiversidad de aves. Bol Soc Biol Concep. 2001;72:109–19.
Rees WE. Urban ecosystems: the human dimension. Urban Ecosyst. 1997;1:63–75.
Romero H, Toledo Órdenes F, Vásquez A. Ecología urbana y gestión ambiental sustentable de las ciudades intermedias chilenas. Ambiente y Desarrollo. 2001;17(4):45–51.
Shanahan DF, Miller C, Possingham HP, Fuller RA. The influence of patch area and connectivity on avian communities in urban revegetation. Biol Conserv. 2012;144:722–9.
Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd ed. New York: WH Freeman; 1995.
Sol D, Bartomeus I, González-Lagos C, Pavoine S. Urbanisation and the loss of phylogenetic diversity in birds. Ecol Lett. 2017;20:721–9.
Solari ME, Cueto C, Hernández F, Rojas JF, Camus P. Procesos territoriales y bosques en la cuenca del río Valdivia (siglos XVI-XIX) (Processes land and forests in the Valdivia River basin). Rev Geogr Norte Gd. 2011;49:45–62.
Solbrig O. Biodiversity: an introduction. In: Solbrig OT, van Emden HM, van Oordt P, editors. Biodiversity and global change. Wallingford: CAB International; 1994.
Steiner F. Landscape ecological urbanism: origins and trajectories. Landsc Urban Plan. 2011;100:333–7.
Tykac J. Arbustos ornamentales. Madrid: Ediciones Susaeta; 1993.
Urquiza A, Mella J. Riqueza y diversidad de aves en parques de Santiago durante el período estival. Bol Chil Ornitol. 2002;9:12–21.
Vejrup P. Caracterización del sur de la ciudad de Buenos Aires, Argentina [Characterization of Southern Buenos Aires, Argentina]. Thesis. Universidad de Flores, Argentina. 2004.
Verner J, Milne KA. Coping with sources of variability when monitoring population trends. Ann Zool Fenn. 1989;26:191–9.
de Vivar J. Crónica y relación copiosa y verdadera de los Reinos de Chile (1558) [Chronicle and abundant and true relation of the Kingdoms of Chile]. Berlin: Edición de Leopoldo Sáez Godoy, Biblioteca Ibero–americana, Berlin Colloquium Verlag; 1979.
White JG, Antos MJ, Fitzsimons JA, Palmer GC. Non-uniform bird assemblages in urban environments: the influence of streetscape vegetation. Landsc Urban Plan. 2005;71:123–35.
Willson M, Armesto J. Efectos de la fragmentación de bosques para las aves de los bosques australes chilenos. Ambiente y Desarrollo. 2003;19:54–9.
Willson MF, De Santo TL, Sabag C, Armesto JJ. Avian communities of fragmented south-temperate rainforests in Chile. Conserv Biol. 1994;8:508–20.
Zar JH. Biostatistical analysis. 5th ed. New Jersey: Prentice Hall Inc; 2010.
Zhoua D, Fungb T, Chua LM. Avian community structure of urban parks in developed and new growth areas: a landscape–scale study in Southeast Asia. Landsc Urban Plan. 2012;108:91–102.
Authors’ contributions
AMP, MGU designed the experiment; MGU participated in the field work; MGU, AMP, FE, HVN carried out the analyses; AMP, MGU drafted the manuscript and HVN, FE reviewed it. All authors read and approved the final manuscript.
Acknowledgements
We thank to J. Urrutia for his help in field work.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All the fieldwork was conducted in accordance with Chilean laws that protect wild flora and fauna.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Muñoz-Pedreros, A., González-Urrutia, M., Encina-Montoya, F. et al. Effects of vegetation strata and human disturbance on bird diversity in green areas in a city in southern Chile. Avian Res 9, 38 (2018). https://doi.org/10.1186/s40657-018-0130-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40657-018-0130-9