Abstract
Background
Radium-223, a targeted alpha therapy, is used to treat symptomatic patients with castration-resistant prostate cancer (CRPC) and bone metastases. Data for radium-223 in asymptomatic CRPC patients with bone metastases are lacking.
Methods
This was a prospective, single-arm phase 3b study. Patients with metastatic CRPC (malignant lymphadenopathy not exceeding 6 cm was allowed, visceral disease was excluded) received radium-223, 55 kBq/kg intravenously, every 4 weeks for up to 6 cycles. Co-primary endpoints were safety and overall survival. Post hoc analyses were performed according to baseline asymptomatic or symptomatic disease status. Asymptomatic status was defined as no pain and no opioid use at baseline.
Results
Seven hundred eight patients received ≥1 radium-223 injection: 548 (77%) were symptomatic to various degrees, and 135 (19%) were asymptomatic. Asymptomatic patients had more favorable baseline disease characteristics than symptomatic. A lower proportion of asymptomatic versus symptomatic patients had received prior abiraterone (25% vs 35%) and prior docetaxel (52% vs 62%). A higher proportion of asymptomatic (71%) versus symptomatic (55%) patients completed radium-223 treatment. Overall survival (hazard ratio [HR] 0.486), time to disease progression (HR 0.722) and time to first symptomatic skeletal event (HR 0.328) were better in asymptomatic than symptomatic patients. Alkaline phosphatase (ALP) response rates were similar (46% vs 47%), and ALP normalization (44% vs 25%) and prostate-specific antigen response rates (21% vs 13%) were higher in asymptomatic than symptomatic patients. A lower proportion of asymptomatic patients reported treatment-emergent adverse events (TEAEs, 61% vs 79%), grade 3–4 TEAEs (29% vs 40%) and drug-related TEAEs (28% vs 44%). There were two treatment-related deaths, both in patients with baseline symptomatic disease.
Conclusions
Using radium-223 earlier in the disease course, when patients are asymptomatic or minimally symptomatic, may enable patients to complete treatment and optimize treatment outcome compared to symptomatic patients, and therefore may allow sequencing with other life-prolonging therapies.
Trial registration
The study was registered with ClinicalTrials.gov, number NCT01618370 on June 13, 2012 and the European Union Clinical Trials Register, EudraCT number 2012–000075-16 on April 4, 2012.
Similar content being viewed by others
Background
Radium-223 dichloride (radium-223), a targeted alpha therapy, is incorporated into newly formed bone in areas of osteoblast activity and increased bone turnover surrounding prostate cancer bone metastases [1, 2]. Radium-223 emits high energy alpha particles over a short range resulting in a localized potent antitumor effect and inhibition of tumor-induced osteoblastic activity in preclinical models [3].
Radium-223 is recommended for the treatment of patients with mCRPC and symptomatic bone metastases [4, 5]. In the pivotal phase 3 ALSYMPCA study, patients with mCRPC and symptomatic bone metastases assigned to radium-223 with best standard of care (BSoC) demonstrated prolonged overall survival (median 14.0 vs 11.2 months, hazard ratio [HR] 0.70; 95% CI, 0.58–0.83; p < 0.001) and delayed time to first symptomatic skeletal event (SSE) compared with those assigned to placebo with BSoC [6, 7]. Radium-223 was generally well tolerated and improvements in patient quality of life were reported compared with patients treated in the placebo arm [6, 8].
In the phase 3 study patients with symptomatic bone metastases were defined as those who regularly used analgesic medication (opioids or non-opioids were allowed), or those who were pain-free, but had received external beam radiation therapy (EBRT) for cancer-related bone pain within a 12 week period before enrollment [6]. However, patients with mCRPC and bone predominant disease often initially present without symptoms [9] and data for radium-223 in these patients are lacking.
Data from clinical trials investigating life-prolonging agents have shown that treating patients with mCRPC earlier in their disease course, for example those who are mildly symptomatic or asymptomatic, can be beneficial. In the TAX 327 study docetaxel every 3 weeks compared with mitoxantrone demonstrated an overall survival benefit (HR 0.76) in patients with mCRPC, which was also demonstrated in a subgroup analysis of patients without pain at baseline (HR 0.73) [10]. Androgen receptor axis-targeted agents, abiraterone acetate (abiraterone, HR 0.65) [11] and enzalutamide (HR 0.63) [12], provided a significant survival benefit in comparison with placebo in patients with mCRPC progressing on docetaxel. A similar overall survival benefit was reported in subgroup analyses of patients with no clinically significant baseline pain (abiraterone HR 0.64, and enzalutamide HR 0.59). Moreover, abiraterone (HR 0.81) and enzalutamide (HR 0.71) demonstrated a survival advantage over placebo when administered first-line in asymptomatic or mildly symptomatic patients with mCRPC [13, 14]. Similarly, it may also be the case that radium-223 is beneficial to patients with asymptomatic bone predominant metastases.
In a phase 3b international early access program (iEAP), patients with asymptomatic mCRPC were included [15], which enabled the current analysis investigating the safety and activity of radium-223 in this patient population.
Methods
Study design and treatment
Study design and patient inclusion and exclusion criteria for this phase 3b study have been previously reported in detail [15]. Patients were ≥ 18 years or older, had histologically or cytologically confirmed progressive bone-predominant mCRPC with two or more skeletal metastases on imaging (symptomatic or asymptomatic), and no visceral disease (lymph node-only metastases not exceeding 6 cm were allowed) [15].
Patients were treated with intravenous injections of radium-223, 55 kBq/kg, every 4 weeks for up to 6 cycles [15]. Concomitant treatment was permitted including abiraterone or enzalutamide, and bone supportive agents as previously described [15]. Supportive care was delivered according to local institutional guidelines [15].
Study assessments
Primary endpoints were safety and overall survival. Symptomatic disease was defined as having pain, or using opioids for cancer related pain at baseline. Asymptomatic disease was defined as no pain (Brief Pain Inventory Short Form [BPI-SF] score of 0) and no opioid use at baseline (use of non-opioid analgesics was allowed). Pain severity was assessed using the self-administered validated BPI-SF questionnaire as previously described [15]. Exploratory efficacy variables included time from start of therapy to first SSE, time to disease progression, and alkaline phosphatase (ALP) response and normalization and prostate specific antigen (PSA) response.
SSEs were defined as the use of EBRT to relieve skeletal symptoms, or the occurrence of new symptomatic pathological bone fractures (vertebral or non-vertebral), or the occurrence of spinal cord compression, or a tumor-related orthopedic surgical intervention. Time to first SSE was defined as time in months from the start of radium-223 until occurrence of the first SSE during the study period. Time to disease progression was defined as the time in months from the start of radium-223 to the date that disease progression (including radiographic, clinical and PSA progression) was assessed as per the local standard of care. Total-ALP and PSA responses, and ALP normalization, were defined as previously reported [15]. Specifically responses were defined as ≥30% reduction of the blood level, compared to the baseline value, confirmed by a second value obtained approximately 4 or more weeks later. Total-ALP normalization was defined as the return of the total-ALP value to within the normal range at 12 weeks after the start of treatment, based on 2 consecutive measurements (at least 2 weeks apart), in patients who had their total-ALP above the upper limit of normal (ULN) at baseline [15].
Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 17.1 and graded using the Common Terminology Criteria for Adverse Events version 4.03, as previously reported [15]. Treatment-emergent adverse events (TEAEs) were defined as those occurring on or after the date and time of administration of the first dose of study drug, or if they were present prior to the administration of the first dose of study drug and increased in severity during the study.
Statistics
Exploratory safety and efficacy analyses were performed in patients who had received at least one dose of study drug and for whom symptom status at baseline could be defined. Kaplan-Meier methodology was used to estimate time-to event data. HRs were calculated using a Cox regression model [15]. The HR (asymptomatic vs symptomatic) was calculated using a Cox regression model.
Results
Patients
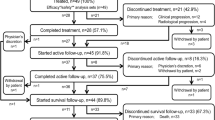
In this updated analysis of the iEAP, 708 patients received at least one radium-223 injection, of whom 683 patients could be defined by symptom status at baseline; 135 (19%) were asymptomatic and 548 (77%) were symptomatic. Twenty-five (4%) patients were excluded from the analysis as symptom status could not be confirmed for reasons including missing baseline pain scores or use of opioids for non-cancer-related pain. Of the asymptomatic patients, 19/135 (14%) only reported use of non-opioid analgesics at baseline.
Patients who were asymptomatic had more favorable baseline characteristics than those who were symptomatic, including Eastern Cooperative Oncology Group performance status (ECOG PS), lower PSA levels and longer time to metastases from initial diagnosis of prostate cancer (Table 1). A lower proportion of patients who were asymptomatic compared with symptomatic had received prior abiraterone (25% vs 35%) and prior docetaxel (52% vs 62%). A higher proportion of patients who were asymptomatic (71%) compared with symptomatic (55%) received all 6 planned cycles of radium-223 (Table 2). The most common reasons for treatment discontinuation in symptomatic (156/248, 63%) compared with asymptomatic (17/39, 43%) patients were those associated with disease progression [see Additional file 1].
During the treatment period for radium-223, comparing asymptomatic with symptomatic patients, 21% vs 16% were treated with concomitant abiraterone, 6% vs 5% with enzalutamide, 18% each with bisphosphonates, and 19% v 18% with denosumab concomitantly.
Efficacy
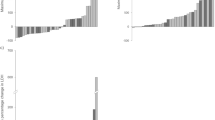
Median follow-up was 9.8 months for both asymptomatic patients (95% CI 8.5–10.9) and for symptomatic patients (95% CI 9.2–10.5). High censoring rates were noted for overall survival and time to first SSE analyses due to the short follow-up time of the trial. Longer medians for overall survival (Fig. 1) and time to disease progression (Fig. 2) were observed in asymptomatic patients compared with those who were symptomatic. HRs of 0.486 (Fig. 1) and 0.722 (Fig. 2) indicated that the risk of death or disease progression was lower by 51 and 28% respectively in the asymptomatic compared with the symptomatic group.
During the study period, SSEs were recorded in 13/135 (10%) patients who were asymptomatic compared with 130/548 (24%) who were symptomatic at baseline [see Additional file 2]. Median time to first SSE was not reached in either of the two groups. The HR of 0.328 indicated that the risk of a SSE was lower by 67% in the asymptomatic compared with the symptomatic group (Fig. 3).
Total-ALP responses [see Additional file 3] were similar in asymptomatic (62/135, 46%) and symptomatic patients (259/548, 47%), as were total-ALP responses in those patients with baseline levels above the ULN (43/71, 61% vs 212/342, 62%). For patients with total-ALP levels above the ULN at baseline, total-ALP was more often returned to the normal range in asymptomatic patients (31/71, 44%) compared with symptomatic patients (86/342, 25%).
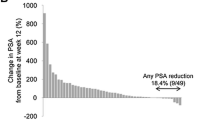
The PSA response rate [see Additional file 4] was higher in asymptomatic (29/135, 21%) compared with symptomatic patients (72/548, 13%), as was the PSA response rate in those patients with baseline PSA levels above the ULN (28/129, 22% vs 65/514, 13%).
Safety
A lower proportion of asymptomatic patients (82/135, 61%) reported TEAEs compared with symptomatic patients (435/548, 79%) (Table 3). The most common grade 3–4 TEAEs (≥3% in either subgroup) in asymptomatic vs symptomatic patients were anemia, 6 (4%) vs 73 patients (13%), thrombocytopenia 2 (1%) vs 22 (4%) back pain 1 (< 1%) vs 19 (3%), bone pain, 3 (2%) vs 26 (5%) and spinal cord compression 0 vs 19 (3%) TEAEs considered to be related to treatment were reported in 38 (28%) asymptomatic patients and 243 (44%) symptomatic patients [see Additional file 5]; the most common grade 3–4 adverse event related to treatment was anemia in 3 (2%) vs 28 (5%) patients respectively. Serious adverse events were reported in 30 (22%) patients with asymptomatic and 210 (38%) patients with symptomatic disease, which in 2 (1%) and 31 patients (6%) respectively were considered to be treatment-related. Adverse events leading to permanent discontinuation were reported in 21 (16%) patients with asymptomatic disease and 123 (22%) with symptomatic disease: in 6 (4%) and 33 (4%) patients respectively they were considered to be related to treatment. Adverse events leading to death were reported in 2 (< 1%) asymptomatic patients (1 cardiac failure and 1 sepsis) and 32 (6%) symptomatic patients (most commonly due to general physical health deterioration in 12 patients). Two TEAEs leading to death were considered to be treatment-related, both in symptomatic patients (1 patient with neutropenia, and 1 with an intestinal perforation).
Discussion
Some clinicians would consider treating patients with mCRPC and asymptomatic disease with radium-223 in their current clinical practice [16]. It may be that administering radium-223 to patients earlier in their disease course, before the onset of severe symptoms and patient clinical deterioration, would lead to improved outcomes. In this iEAP, the safety profile of radium-223 appeared to be better in patients with asymptomatic mCRPC compared with symptomatic disease, with no unexpected adverse events reported in either group. This included fewer TEAEs that were considered to be related to treatment in the asymptomatic group, despite a higher proportion of patients completing 6 radium-223 cycles compared with symptomatic patients. Clinical outcome was also better in radium-223 treated patients with asymptomatic disease who experienced longer overall survival, longer time to disease progression, and a lower risk of SSEs during the study compared with those treated with radium-223 with symptomatic disease. Furthermore, whilst ALP response was similar between the groups, a higher proportion of asymptomatic patients experienced ALP normalization, and PSA responses, compared with symptomatic patients treated with radium-223. Changes in PSA levels are not considered to be a reliable maker for monitoring radium-233 efficacy in this setting [17]. The current findings of a higher PSA response in patients with asymptomatic bone metastases require validation and further investigation in prospective studies.
In the ALSYMPCA study, 513 out of 921 (56%) randomly assigned patients had recorded opioid use at baseline (345 assigned to radium-223 and 168 to placebo). In a subgroup analysis, radium-223 compared with placebo improved overall survival and reduced the risk of initial SSEs during the study, regardless of baseline opioid use [18]. Indeed, radium-223 appeared to be more effective in delaying SSEs in the minimally symptomatic (WHO ladder pain score 0–1) patients who did not require opioid use at baseline (HR: 0.56, 95% CI: 0.39–0.82) compared with those patients with more advanced symptomatic disease (WHO ladder pain score 2–3) who required baseline opioid therapy (HR: 0.72, 95% CI: 0.53–0.98). Furthermore compared with placebo, radium-223 prolonged time to first opioid use and EBRT for pain in the non-opioid group of patients. Radium-223 was well tolerated in patients irrespective of their opioid use at baseline. The authors concluded that pain symptom severity should not be the basis for determining appropriate timing of radium-223 treatment [18].
In this iEAP, patients with asymptomatic disease had more favorable baseline factors that are associated with good prognosis [15, 19, 20], including lower median ALP and PSA levels and lower ECOG PS, suggesting that they had generally less advanced disease than those who had symptoms. Further, the longer time between cancer diagnosis and appearance of bone metastases recorded in the asymptomatic patients may be indicative of a slower disease course in this subgroup of patients. In a separate post hoc analysis of the iEAP, the likelihood of completing radium-223 treatment (receiving 5–6 radium-223 injections) was increased in patients with more favorable prognostic factors at baseline (less pain, low ECOG PS, low PSA level and high hemoglobin level) [21]. In the analysis, overall survival was reported to be longer in patients who received 5–6 injections of radium-223 compared with those who discontinued radium-223 early (received only 1–4 injections of radium-223) [21]. Similar findings were reported from a post hoc analyses of a US EAP and the ALSYMPCA study [22].
Patients treated in this iEAP were generally similar to those currently treated in routine clinical practice, and included chemotherapy-naïve patients, and those who had previously received or were receiving concomitant treatment with abiraterone or enzalutamide [15]. This contrasts with the ALSYMPCA study where patients previously treated with chemotherapy or those who were ineligible for chemotherapy treatment were recruited, but at the time of the ALSYMPCA study abiraterone and enzalutamide were investigational agents and were therefore unavailable [6].
During the treatment period, comparing asymptomatic with symptomatic patients, 21% vs 16% were treated with concomitant abiraterone and 6% vs 5% with concomitant enzalutamide respectively. It is important to note that concomitant treatment of these patients with radium-223 and new hormonal agents may have affected the outcome observed [15]. As similar proportions of asymptomatic vs symptomatic patients received concomitant abiraterone or enzalutamide, we believe that concomitant treatment alone is unlikely to account for the difference in overall survival (medians 20.5 vs 13.5 months) observed between these patient groups.
A phase 3 randomized, double-blind study of radium-223 or placebo, each in combination with abiraterone plus prednisone in chemotherapy-naive patients with asymptomatic or mildly symptomatic mCRPC with bone metastases (ERA 223; NCT02043678) was recently prematurely unblinded. The independent data monitoring committee recommended unblinding the trial due to the observation of more fractures and deaths in the combination treatment arm. Given these results from the ERA 223 trial, the current recommendation is not to combine radium-223 with concomitant abiraterone acetate and prednisone in this asymptomatic patient population [23]. The phase 3 PEACE III trial evaluating radium-223 in combination with enzalutamide versus enzalutamide alone, in patients with mildly symptomatic or asymptomatic mCRPC, is ongoing.
In this single-arm iEAP, the association between symptoms and overall survival confirms the prognostic value of patient symptoms at baseline. Using radium-223 earlier in the disease course, when patients are asymptomatic or minimally symptomatic, may enable patients to complete treatment and optimize treatment outcome compared to symptomatic patients, and therefore may allow sequencing with other life-prolonging therapies.
Conclusion
In conclusion, use of radium-223 in this group of 135 asymptomatic patients seems to be safe in the setting of this iEAP, however, caution is still warranted in daily clinical practice, as the subgroup size from this study was small, the drug is not approved in this setting, and the final results of prospective studies have to be awaited.
Abbreviations
- ALP:
-
Alkaline phosphatase
- BPI-SF:
-
Brief Pain Inventory Short Form
- BSoC:
-
Best standard of care
- CRPC:
-
Castration-resistant prostate cancer
- EBRT:
-
External beam radiation therapy
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- HR:
-
Hazard ratio
- iEAP:
-
International early access program
- mCRPC:
-
Metastatic castration-resistant prostate cancer
- MedDRA:
-
Medical Dictionary for Regulatory Activities
- PSA:
-
Prostate specific antigen
- SSE:
-
Symptomatic skeletal event
- TEAEs:
-
Treatment-emergent adverse events
- ULN:
-
Upper limit of normal
References
Henriksen G, Breistol K, Bruland OS, et al. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002;62:3120–5.
Bruland OS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12:6250s–7s.
Suominen MI, Fagerlund KM, Rissanen JP, et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models. Clin Cancer Res. 2017;23:4335–46.
Parker C, Gillessen S, Heidenreich A, et al. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v69–77.
NCCN guidelines-Prostate cancer version 1. 2018. https://www.nccn.org.
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–46.
Nilsson S, Cislo P, Sartor O, et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol. 2016;27:868–74.
Drudge-Coates L, Oh WK, Tombal B, et al. Recognizing symptom burden in advanced prostate Cancer: a global patient and caregiver survey. Clin Genitourin Cancer. 2018;16:e411–9.
Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5.
de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60.
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–16.
Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2018;73:178–211.
Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–7.
Parker C, Finkelstein SE, Michalski JM, et al. Efficacy and safety of Radium-223 dichloride in symptomatic castration-resistant prostate Cancer patients with or without baseline opioid use from the phase 3 ALSYMPCA trial. Eur Urol. 2016;70:875–83.
Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27:454–60.
Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–7.
Saad F, Keizman D, O'Sullivan JM, et al. Analysis of overall survival by number of radium-223 injections received in an international expanded access program (iEAP). J Clin Oncol. 2016;34(Suppl 15):5082.
Sartor O, Coleman RE, Morris MJ, et al. Baseline characteristics, number of radium-223 (Ra-223) injections, and overall survival (OS) in US expanded access program (EAP) and ALSYMPCA. Eur J Cancer. 2015;51(Suppl 3):S484–5.
Euorpean Medicines Agency: Warning about use of prostate cancer medicine Xofigo in combination with Zytiga and prednisone or prednisolone http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Xofigo/human_referral_prac_000071.jsp&mid=WC0b01ac05805c516f&source=homeMedSearch&category=human. Accessed 18 Jan 2018.
Acknowledgements
Paul Hoban PhD of Cancer Communications and Consultancy Ltd., Knutsford UK, provided medical writing assistance which was funded by the Pharmaceutical Division of Bayer. These data have previously been presented in part at the American Society of Clinical Oncology Genitourinary Cancer Symposium, Orlando FL February 16-18 2017. https://meetinglibrary.asco.org/record/140521/poster
Funding
The phase 3b iEAP study was sponsored by the Pharmaceutical Division of Bayer. The funder was responsible for the study design, data management and statistical analysis. Data interpretation was performed in collaboration with the study steering committee and the authors. The funder supported development of the manuscript through provision of medical writing assistance. FS had full access to all the study data and all authors had the final responsibility for the decision to submit for publication.
Availability of data and materials
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Author information
Authors and Affiliations
Contributions
AH, SG, JC, MW, MS, SN and FS were involved in the study concept and design; DH, DK, JO’S, MW, and FS were involved in acquisition of data; AH, SG, DH, DK, JO’S, JC, MW, KM, JR, MS, SN and FS were involved in analysis and interpretation of data; AH, SG, DH, JO’S, JC, MW, MS, SN, and FS were involved in manuscript drafting or critical revision of its content. DK and JR reviewed the manuscript. All authors approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol and all protocol amendments were reviewed and approved by each study site’s Independent Ethics Committee/Institutional Review Board before the start of the study (listed below). All patients provided written informed consent prior to entry into the international, prospective, interventional, open-label, single-arm, phase 3b study that was conducted in compliance with the Declaration of Helsinki, International Conference on Harmonisation Guidelines for Good Clinical Practice and all local legal and regulatory requirements.
Local ethics committees (central ethics committees) — Belgium: (Central) CU Saint-Luc/UZ St-Luc, Comité d’Ethique Hospitalo-Facultaire, Brussels (trial unit [TU] 28,001); Institut Jules Bordet/Jules Bordet Instituut Comité Ethique/Ethisch Comité, Brussels (TU 28002); UZ Gent Ethisch Comité, Gent (TU 28003); UZ Leuven Gasthuisberg Commissie voor Medische Ethiek/klinisch Onderzoek, Leuven (TU 28007); UZ Brussel Comité Ethique/Ethisch Comité, Brussels (TU 28009). Canada: Princess Margaret Hospital-University Health Network, University Health Network Research Ethics Board, Toronto (TU 26004); British Columbia Cancer Agency-Vancouver Centre Research Ethics Board, Vancouver (TU 26005); Sunnybrook Health Sciences Centre, Sunnybrook Research Ethics Board Research Ethics Office, Toronto (TU 26006); Ottawa Health Science Network Research Ethics Board, Ottawa (TU 26007); CHUM - Hopital Notre-Dame, Comité d’évaluation scientifique et d’éthique de la recherche du CHUM, Montreal (TU 26008). Finland: (Central) HUS Tutkimuseettiset toimikunnat Operatiivinen eettinen toimikunta Biomedicum, Helsinki (TU 59002, 59,003, 59,004, 59,005). Germany: (Central) Landesamt für Gesundheit und Soziales Geschäftsstelle der Ethik-Kommission des Landes, Berlin (TU 10001, 10,014, 10,025); Fakultät Carl Gustav Carus, Ethik-Kommission der Technischen Universität Dresden, Dresden (TU 10002); Universitätsklinikum Aachen Ethik-Kommission, Aachen (TU 10003); Ethik-Kommission des Fachbereichs Medizin der Johann Wolfgang Goethe-Universität, Frankfurt (TU 10006); Klinikum der Universität München Großhadern, Ethikkommission der LMU, Munich (TU 10007); Ethikkommission der Fakultät für Medizin der Technischen Universität München, Munich (TU 10010); Ethik-Kommission der Landesärztekammer Rheinland-Pfalz, Mainz (TU 10011); Universität Rostock - Medizinische Fakultät Ethikkommission an der Medizinischen Fakultät, Rostock (TU 10013); Ethikkommission des Landes Bremen Institut für Klinische Pharmakologie Klinikum Bremen-Mitte gGmbH, Bremen (TU 10016); Universitätsklinikum Erlangen Ethik-Kommission der Medizinischen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen (TU 10017); Medizinische Medizinische Fakultät der Otto-von-Guericke Universität Ethikkommission, Magdeburg (TU 10018); Ethik-Kommision der Medizinischen Fakultät der Eberhard-Karls-Universität und am Universitätsklinikum Tübingen, Tübingen (TU 10019); Ethik-Kommission der Friedrich-Schiller-Universität Jena, Jena (TU 10020); Ethikkommission an der Medizinischen Fakultät der Heinrich-Heine-Universität, Düsseldorf (TU 10021); Ethik-Kommission des Fachbereichs Medizin der Philipps-Universität Marburg (TU 10026); Ethik-Kommission der Landesärztekammer Baden-Württemberg, Stuttgart (TU 10027); Universitätsklinikum Ulm Ethik-Kommission, Ulm (TU 10029); Ethik-Kommission der Ärztekammer Hamburg, Hamburg (TU 10030). Ireland: Clinical Research Ethics Committee of the Cork Teaching Hospital, Cork (TU 80001, 80,002, 80,003). Israel: Soroka University Medical Center Ethics Committee, Beer Sheva (TU 39001); Meir Medical Center Ethics Committee, Kafar Saba (TU 39002); Ethic Committee, Rambam Medical Center Tamar, Hafia (TU 39003); Assaf Harofeh Medical Center Ethics Committee, Zrifin (TU 39004); Hadassah Hebrew University Hospital Ein Kerem Helsinki Committee, Hadassah University Medical Center, Jerusalem (TU 39005); Rabin Medical Center - Beilinson Campus Ethics Committee, Petah Tikva (TU 39006); Tel-Aviv Sourasky Medical Center Ethics Committee, Tel-Aviv (TU 39007); Chaim Sheba Medical Center Ethics Committee, Ramat Gan (TU 39009). Italy: IRST Istituto Scientifico Romagnolo per studio e cura Tumori CE IRST IRCCS-AVR: Comitato Etico IRST IRCCS e Area Vasta Romagna, Forli (TU 22001); Fondazione IRCCS Istituto Nazionale dei Tumori di Milano Comitato Etico Centrale IRCCS Lombardia sezione IRCCS Istituto Nazionale Tumori, Milano (TU 22002); A.O.U. Pisana Ceavno: Comitato Etico di Area Vasta Nord Ovest Toscana, Pisa (TU 22005); IRCCS Istituto Clinico Humanitas - Humanitas Mirasole S.p.A. Comitato Etico IRCCS Lombardia Sezione Istituto Clinico Humanitas, Milano (TU 22007); Azienda Policlinico Umberto I Comitato Etico Universita’ La Sapienza, Roma (TU 22008); A.O.U. Policlinico G. Martino Comitato Etico Interaziendale Provincia Di Messina, Messina (TU 22009); A.O.U. San Luigi Gonzaga CEI: Comitato Etico Interaziendale A.O.U. San Luigi Gonzagsa e ASL TO2 TO3 TO4 TO5, Orbassano, Torino (TU 22010); E.O. Ospedali Galliera Comitato Etico Regione Liguria Sezione 2 Sperimentazioni Adulti, Genova (TU 22012); AULSS 09 Treviso CESC: Comitato Etico Sperimentazione Clinica Province Trevisobelluno, Treviso (TU 22017); Comitato Etico Di Area Vasta Sud Est Toscana - Sezione di Arezzo, Arezzo (TU 22018); APSS Trento – Trentino Comitato Etico Sperimentazioni Cliniche Azienda Provinciale per i Servizi Sanitari (APSS), Trento (TU 22021). A.O. Sant’Andrea Comitato Etico Universita’ La Sapienza, Roma (TU 22028): A.O. San Camillo-Forlanini Comitato Etico Lazio 1, Roma (TU 22029). Norway: (Central) Regional komité for medisinsk og helsefaglig forskningsetikk, Oslo (TU 36001, 36,002, 36,003, 36,004, 36,006); Netherlands: (Central) Commissie Mensgebonden Onderzoek (CMO) UMC St-Radboud, Nijmegen (TU 30003, 30,004). Poland: Komisja Bioetyczna przy Centrum Onkologii Centrum Onkologii - Instytut im. M. Sklodowskiej-Curie, Warszawa (TU 18001, 18,006). Russian Federation: National Medical Research Radiology Center, Local ethical committee for clinical trials Medical Radiological Research Centre of RAMS, Obninsk (TU 51002); EC at Oncological Scientific Center n.a N.N. Blokhin 24, Kashirskoe shosse, Moscow (TU 51003). Spain: (Central) Ciutat Sanitària i Universitaria de la Vall d’Hebron Comité Étic d’Investigació Clínica, Barcelona (TU 24001); Clínica Universidad de Navarra CUN, Comité Ético de Investigación Clínica de Navarra, Pamplona (TU 24002); Hospital General Universitario Gregorio Marañón Comité Ético de Investigación Clínica Secretaría CEIC, Madrid (TU 24008); CEIC Hospital Ramón y Cajal, Madrid (TU 24004); Hospital Clínico Universitario de Santiago de Compostela Comité Etico de Investigación Clínica SERGAS, Santiago de Compostela (TU 24006); CEIC Investigación Biomédica de Andalucían, Consejeria de Salud Secretaria General de Calidad y Eficiencia, Sevilla (24,014, 24,028); Hospital Virgen de la Victoria Comité Etico de Investigación Clínica, Malaga (TU 24007); Fundación Hospital Alcorcón Comité Ético de Investigación Clínica, Alcorcón (TU 24009); Hospital de la Santa Creu i de Sant Pau Comité Ético de Investigación Clínica, Barcelona (TU 24010); Hospital Clínic i Provincial de Barcelona Comité Ético de Investigación Clínica, Agencia de Ensayos Clínicos, Barcelona (TU 24011); Hospital Universitari Son Espases Comité Ético de Investigación Clínica de las Islas Baleares Conselleria de Salut i Consum, Palma de Mallorca (TU 24012); Hospital Universitario “La Paz” CEIC Área 5 - H.U. La Paz, Madrid (TU 24013); Comité Ético de Investigación Clinica de Navarra, Hospital de Navarra, Pamplona (TU 24015); Ciutat Sanitària i Universitària de Bellvitge, Comité Ético de Investigación Clínica Secretaria Administrativa/Unitat de Recerca, L’Hospitalet de Llobregat, Barcelona (TU 24017); Hospital Universitario “Marqués de Valdecilla” Comité Etico de Investigación Clínica, Santander (TU 24023); Hospital Universitario 12 de Octubre Comité Ético de Investigación Clínica, Madrid (TU 24024); Hospital Central de Asturias Secretaría del Comité Ético de Investigación Clínica, Oviedo (TU 24032); Hospital Universitari i Politècnic La Fe Comité Etico de Investigación Clínica, Valencia (TU 24033). Sweden: (Central) Etikprövningsnämnden Regionala etikprövningsnämnden i Stockholm, Karolinska Institutet, Stockholm (TU 34001–34,009, 340,011). Switzerland: Commission cantonale d’éthique de la recherche sur l’être humain, Lausanne (TU 58001); Ethikkommission St. Gallen, Kantonspital St. Gallen, St. Gallen (TU 58002); Kantonale Ethikkommission Zürich, Zürich (TU 58003); Kantonale Ethikkommission Aargau, Departement Gesundheit und Soziales, Aarau (TU 58004); Ethikkommission NordWest- und Zentralschweiz, Basel (TU 58005); Kantonale Ethikkommission Bern – KEK Institut für Pathophysiologie, Bern (TU 58007); Commission cantonale d’éthique de la recherche CCER, Genève (TU 58009). United Kingdom: National Research Ethics Service (NRES) Committee South West – Central Bristol South West Research Ethics Committee Centre, Bristol (TU 12006, 12,011, 12,015, 12,019, 12,020, 12,021, 12,024, 12,026).
Consent for publication
Not applicable.
Competing interests
A.H. has received honoraria from Amgen, Astellas, Bayer, Dendreon, Ferring, Ipsen, Jansen, Pfizer, Sanofi, Takeda and has received research funding from Amgen, Astellas, and Sanofi; S.G. has compensated consultancy/advisory roles with AAA International, Active Biotech AB IDMC, Astellas Pharma, Bayer, Bristol-Myers Squibb, Clovis, Curevac, Dendron Corporation, Ferring, Innocrin Pharmaceuticals, Janssen-Cilag, MaxiVAX SA, Millennium, Pharmaceuticals, Novartis, Orion, Pfizer, Roche and Sanofi Aventis, and has participated in Speakers Bureaus (compensated) for Janssen and Novartis and has a patent application for a biomarker method (WO 2009138392 A1); D.H. has received honoraria from Janssen-Cilag, Astellas and Bayer, has compensated consultancy/advisory roles with Astellas, Bayer and Amgen and has had travel/accommodation expenses reimbursed from Bayer; D.K. has received honoraria from and has compensated consultancy/advisory roles with Pfizer, Sanofi, Bayer and Novartis and has had travel/accommodation/expenses reimbursed from Pfizer and Bayer; JMO has participated in advisory boards and speaker’s bureaus for Bayer, Janssen, Sanofi and has received research funding (institute) from Bayer; JC has a compensated consultancy role and has participated in scientific advisory boards for Johnson & Johnson, Astellas, Bayer, Amgen, Pfizer, BMS and has participated in speaker’s bureaus for Bayer and Johnson & Johnson; MW has compensated consultancy or advisory roles with ABX, Apogepha, Astellas, Amgen, Janssen-Cilag, Bayer, MSD and has provided expert testimony for ABX; KM has received honoraria from Bayer, Janssen and Amgen and has compensated consultancy/advisory roles with Astellas, Astra Zeneca, Bristol-Myers Squibb, Ferring, Janssen, MSD, Novartis, Roche and Sotio; J.R. and M.S. are salaried employees of Bayer; SN has received honoraria from Bayer, Astellas, Ipsen, Sanofi-Genzyme, Novartis, Roche and Janssen for advisory board participations and lectures; F.S. has a compensated consultancy role with, and has received research funding from, Bayer, Astellas, Janssen and Sanofi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Patient disposition according to symptom status. (DOCX 30 kb)
Additional file 2:
Table S2. SSEs occurring during the study according to symptom status. (DOCX 28 kb)
Additional file 3:
Table S3. Total-ALP response. (DOCX 29 kb)
Additional file 4:
Table S4. PSA response. (DOCX 29 kb)
Additional file 5:
Table S5. Summary of treatment-related adverse events by symptom status. (DOCX 31 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Heidenreich, A., Gillessen, S., Heinrich, D. et al. Radium-223 in asymptomatic patients with castration-resistant prostate cancer and bone metastases treated in an international early access program. BMC Cancer 19, 12 (2019). https://doi.org/10.1186/s12885-018-5203-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-5203-y