Abstract
Background
Only recently, regulations on the names of medicines were developed. Regulations are mainly focused on avoiding the approval of medicine names that may be confusing to others. Furthermore, legal requirements do not include testing for human factors, such as potential users’ preferences.
Study aims
To develop a set of new brand names of medicines, to determine subjects’ preferred names, and to evaluate if the linguistic features of these names were related to subjects’ preferences.
Methods
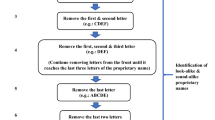
Forty-six new names linguistically equivalent to the Portuguese brand names of medicines were developed. A panel of 13 postgraduates on linguistic studies were purposively enrolled. Participants were required to select and categorize the 6 most preferred names.
Results
From the 29 selected names: 62.1% ended in consonants, 65.5% contained at least one syllable of the CVC type, and 62.1% presented final stress. Considering these 3 linguistic features, there were statistically significant differences between the preferred and underpreferred names: χ2 = 4.572, P =.032; χ2 = 5.599, P =.018; and χ2 = 4.572; P =.032, respectively. Conclusions: Some linguistic features of the evaluated names were related to subjects’ preferences. Tests on subjects’ preferences about the names of medicines may provide additional safety features addressed by the present regulations.
Similar content being viewed by others
References
Corrigan J, Kohn LT, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 1999.
Kenagy JW, Stein GC. Naming, labeling, and packaging of pharmaceuticals. Am J Health Syst Pharm. 2001;58:2033–2041.
Food and Drug Administration. Guidance for industry contents of a complete submission for the evaluation of proprietary names, 2016. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm075068.pdf. Accessed August 13, 2016.
INFARMED, IP. Deliberação no. 144/CD/2012—Norma orientadora para a aceitação de nomes de medicamentos [Resolution no. 144/CD/2012—Guiding norm for the acceptance of the medicines names]. http://www.infarmed.pt/portal/page/portal/INFARMED/MEDICAMENTOS_USO_HUMANO/AUTORIZACAO_DE_INTRODUCAO_NO_MERCADO/144_DAM_91.pdf. Accessed August 13, 2016.
Handler SM, Nace DA, Studenski SA, Fridsma DB. Medication error reporting in long-term care. Am J Geriatr Pharmacother. 2004;2:190–196.
Institute of Medicine. Preventing medication errors, 2007. http://www.nap.edu/catalog.php?%20record_id=11623. Accessed August 13, 2016.
Ostini R, Roughead E, Kirkpatrick C, Monteith G, Tett S. Quality use of medicines—medication safety issues in naming; look alike, sound-alike medicine names. Int J Pharm Pract. 2012;20:349–357.
Lambert B, Lin S, Tan H. Designing safe drug names. Drug Safety. 2005;28:495–512.
Jennifer JA, Monica P, Malcolm CS. The sound of brands. J Market. 2010;74:97–109.
Usunier JC, Shaner J. Using linguistics for creating better international brand names. J Market Commun. 2002;8:211–218.
Shrum LJ, Lowrey TM, Luna D, Lerman D, Liu M. Testing phonetic symbolism effects on brand name preference for bilinguals across multiple languages. In Dahl DW, Johar GV, van Osselaer SMJ, eds. NA—Advances in Consumer Research. Vol. 38. Duluth, MN: Association for Consumer Research.
Meier B, Rey-Mermet A, Rothen N, Graf P. Recognition memory across the lifespan: the impact of word frequency and study-test interval on estimates of familiarity and recollection. Front Psychol. 2013;4:787.
Luna D, Carnevale M, Lerman Dawn. Does brand spelling influence memory? The case of auditorily presented brand names. J Consum Psychol. 2013;23:36–48.
Cavaco A, Costa M, Pires C, Correia S, Vigário M. Exploring memory issues with the brand names of medicines. Int J Pharm Pract. 2016;(suppl 2): 4.
European Medicines Agency. Guideline on the acceptability of names for human medicinal products processed through the center procedure. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004142.pdf. Published 2014. Accessed August 13, 2016.
Citrome L. What’s in a name? Use of brand vs. generic drug names. Int J Clin Pract. 2016;70:3–4.
NHS choices. Medicine information—brand names and generics. http://www.nhs.uk/Conditions/Medicinesinfo/Pages/Brandnamesandgenerics.aspx. Published 2014. Accessed August 13, 2016.
Pires C, Vigário M, Cavaco A. Brand names of Portuguese medicines: understanding the importance of their linguistic structure and regulatory issues. Cien Saude Colet. 2015;20:2569–2583.
Lamber B, Bhamuik R, Zhao W, Bhaumik D. Detection and prediction limits for identifying highly confusable drug names from experimental data. J Biopharm Stat. 2016;26:365–3685.
Schroeder SR, Salomon MM, Galanter WL, et al. Cognitive tests predict real-world errors: the relationship between drug name confusion rates in laboratory-based memory and perception tests and corresponding error rates in large pharmacy chains [published online May 18, 2016]. BMJ Qual Saf. doi:https://doi.org/10.1136/bmjqs-2015-005099.
Food and Drug Administration. Strategies to reduce medication errors: working to improve medication safety. https://www.fda.gov/drugs/resourcesforyou/consumers/ucm143553.htm. Published 2015. Accessed June 3, 2017.
Therapeutics Goods Administration. Best practice guideline on prescription medicine labeling. https://www.tga.gov.au/publication/best-practice-guideline-prescription-medicine-labelling. Published 2011. Accessed August 13, 2016.
INFARMED, IP. Prontuário Nacional Terapêutico–10 [National Prescribing Guide–10]. Lisbon: Autoridade Nacional do Medicamento e Produtos de Saúde, I.P.; 2011.
Mateus MH, Brito AM, Duarte I, et al. Gramática da Língua Portuguesa [Portuguese grammar], 6th ed. Lisbon: Caminho; 2004.
Andrews S. The effect of orthographic similarity on lexical retrieval: resolving neighborhood conflicts. Psychon Bull Ver. 1997;4:439–461.
Lambert BL, Lin SJ, Tan H. Designing safe drug names. Drug Saf. 2005;28:495–512.
New B, Ferrand L, Pallier C, Brysbaert M. Reexamining the world length effect in visual word recognition: new evidence from the English Lexicon Project. Psychon Bull Rev. 2006;13:45–52.
Vigário M, Frota S, Martins F. Para uma caracterização da distinção entre palavras prosódicas e clíticos com base em dados de frequência. In: XXVI Encontro Nacional da Associação Portuguesa de Linguística, 2011. Textos Selecionados. Lisboa: Associação Portuguesa de Linguística, 589603. http://labfon.letras.ulisboa.pt/texts/Vigario_Frota_Martins_2011.pdf.
Frota S, Vigário M, Martins F, Cruz M. FrePOP: frequency patterns of phonological objects in Portuguese (version 1.0), 2007 [Database].
Cooper N, Cutler A, Wales R. Constraints of lexical stress on lexical access in English: evidence from native and non-native listeners. Lang Speech. 2002;45:207–228.
Loken B, Joiner C, Peck J. Category attitude measures: exemplars as inputs. J Consum Psychol. 2002;12:149–161.
Ma Q, Wang C, Wang X. Two-stage categorization in brand extension evaluation: electrophysiological time course evidence. PLoS One. 2014;9:e114150.
Friedman M, Leclercq T. Brand discrimination: an implicit measure of the strength of mental brand representations. PLoS One. 2015;10:e0121373.
Priester JR, Nayakankuppam D, Fleming MA, Godek J. The A 2 SC 2 Model: the influence of attitudes and attitude strength on consideration and choice. J Consum Res. 2004;30:574–587.
Petty RE, Haugtvedt CP. Elaboration as a determinant of attitude strength: creating attitudes that are persistent, resistant, and predictive of behavior. In: Petty RE, Krosnick JA, eds. Attitude Strength: Antecedents and Consequences. New York: Psychology Press; 1995:93–138.
Martins F, Vigário M, Frota S. FreP: Frequency Patterns of phonological objects in Portuguese (version 2.0), 2016 [Software]. http://labfon.letras.ulisboa.pt/FreP/tools.html. Accessed August 13, 2016.
Baddeley A. The magical number seven: still magic after all these years? Psychol Rev. 1994;101:353–356.
Vigário M, Martins F, Frota S. A ferramenta FreP e a frequência de tipos silábicos e de classes de segmentos no Português. [The tool FreP and the frequency of the syllabic types and classes of segments in Porguese]. Paper presented at: XXI National Meeting of the Portuguese Association of Linguistics, 2006.
Mchugh ML. The chi-square test of independence. Biochem Med (Zagreb). 2013;23:143–149.
One World Nations Online. Most common languages. http://www.nationsonline.org/oneworld/most_spoken_languages.htm. Accessed June 6, 2017.
Vigário M, Frota S, Martins F. A frequência que conta na aquisição da fonologia: types ou tokens. In: XXV Encontro Nacional da Associação Portuguesa de Linguística, 2010. Textos selecionados. Porto: Associação Portuguesa de Linguística, 749767. http://labfon.letras.ulisboa.pt/texts/Vigario_Frota_Martins_2010.pdf.
Taylor K, Holquist CA. More on confusion of drug names. N Engl J Med. 2013;368:1946–1947.
Keller KL. Conceptualizing, measuring, and managing customer-based brand equity. J Mark. 1993;57:1–22.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pires, C.M.B.F., Cavaco, A. Design of Brand Names of Medicines Considering Subjects’ Preferences. Ther Innov Regul Sci 52, 230–235 (2018). https://doi.org/10.1177/2168479017719933
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479017719933