Abstract
Background
The US FDA has developed numerous accelerated pathways (APs) to facilitate faster development and approval of innovative drugs addressing unmet needs.
Methods
To gauge how payers in the United States view APs, PAREXEL and the Network for Excellence in Healthcare Innovation (NEHI) surveyed 20 national, regional, public, and private payers whose coverage decisions impact 228 million patients.
Results
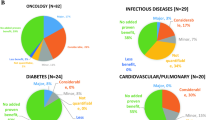
The survey shows that APs have created new challenges and concerns for payers, including greater difficulty valuing drugs that have less clinical information available at launch.
Conclusions
Our survey indicates that policies must further the goal of getting needed new medicines to patients expeditiously, while managing the risks AP drugs entail for payers and, most importantly, patients. For developers, a trend toward value-based assessments of both AP and non-AP drugs by payers may require ever-earlier and more data-driven decision making about the value of new products in development.
Similar content being viewed by others
References
Jaffe S. Robert Califf: leading cardiologist is new FDA Deputy Commissioner. Lancet. 2015;385:765.
United States Food and Drug Administration Novel Drugs Summary 2015. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM481709.pdf. Published January 2016. Accessed January 21, 2016.
United States Government Accountability Office. Drug safety: FDA expedites many applications, but data for postapproval oversight need improvement. http://www.gao.gov/assets/680/674183.pdf. Published December 2015. Accessed February 8, 2016.
Jenkins J. CDER new drug review: 2015 update [slide presentation]. Presented at: FDA/CMS Summit; December 14, 2015; Washington, DC. http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM477020.pdf. Accessed February 8, 2016.
Schwan S. Keynote address. Presented at: Financial Times Global Pharmaceutical and Biotechnology Conference; November 16, 2015; London, England. https://live.ft.com/Events/2015/FT-Global-Pharmaceutical-and-Biotechnology-Conference. Accessed February 8, 2016.
European Medicines Agency. Reflection paper on a proposal to enhance early dialogue to facilitate accelerated assessment of priority medicines (PRIME). http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2015/10/WC500196065.pdf. Published October 22, 2015. Accessed February 8, 2016.
Macaulay R. FDA breakthrough status versus accelerated approval—what’s the difference? Presented at: ISPOR 18th Annual European Congress; November 10, 2015; Milan, Italy. Poster PHP48.
Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175:1992–1994.
Gilead Sciences, Inc. Form 10-Q, May 8, 2015, p. 26, from website of the United States Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/882095/000088209515000017/q115form10-q.htm. Accessed September 12, 2016.
California Technology Assessment Forum. The comparative clinical effectiveness and value of novel combination therapies for the treatment of patients with genotype 1 chronic hepatitis C infection. https://icer-review.org/wp-content/uploads/2016/01/CTAF_HCV2_Final_Report_013015.pdf. Published 2015. Accessed February 8, 2016.
Institute for Clinical and Economic Review. New lower prices for Gilead hepatitis C drugs reach CTAF threshold for high health system value [press release]. http://www.icer-review.org/new-lower-prices-for-gilead-hepatitis-c-drugs-reach-ctaf-threshold-for-high-health-system-value/. Published February 17, 2015. Accessed February 8, 2016.
United States Food and Drug Administration. Drugs@FDA website. https://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed February 8, 2016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farrimond, B., Fleming, J.J. & Mathieu, M. Accelerated Pathways Work—Now What? A Survey of Payers in the United States. Ther Innov Regul Sci 51, 224–231 (2017). https://doi.org/10.1177/2168479016668221
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479016668221