Abstract
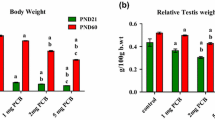
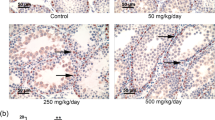
Polychlorinated biphenyl (PCB) is an endocrine-disrupting chemical. Sertoli cells (SCs) provide physical and nutritional support for developing germ cells. Dysfunction in SCs has adverse effects on spermatogenesis. Previously, we found that the lactational exposure of PCBs (1, 2, and 5 mg/kg birth weight/day, orally from postnatal days 1 to 20) decreased the follicle-stimulating hormone receptor (FSHR) and androgen receptor (AR) expression in SCs of F1 progeny. Transcription factors initiate and regulate the transcription of genes. DNA methylation plays an important role in epigenetic gene regulation. Hence, this study was aimed to identify the level of transcription factors regulating FSHR, AR gene expression, and DNA methylation in the promoter of these genes in SCs of both F1 prepuberal and puberal offspring. DNA methylation in the promoter of FSHR and AR genes was examined by sodium bisulfite conversion technique. The protein levels of transcription factors (steroidogenic factor 1 [SF1], upstream stimulatory factors 1 and 2, c-fos, c-jun, and CREB-binding protein) and enzymes DNA methyltransferases (Dnmt1, Dnmt3ab, Dnmt3l, and histone deacetylase 1 [HDAC1]) were analyzed by Western blotting. The transcription factors that regulate the FSHR and AR gene in SCs were decreased in both the PCB-exposed F1 progeny. Methylation was observed in the promoter of FSHR, AR, and SF1. The protein levels of Dnmt1, Dnmt3ab, Dnmt3l, and HDAC1 were increased in the PCBstreated groups. Subsequently, it leads to transcriptional repression of the genes in SCs. Our finding suggests that PCBs caused epigenetic change in SCs, thereby it impaired SCs function in F1 progeny.
Similar content being viewed by others
References
Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli celldepleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122(3):787–794.
Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125(6): 769–784.
Choi MS, Park HJ, Oh JH, Lee EH, Park SM, Yoon S. Nonylphenol-induced apoptotic cell death in mouse TM4 Sertoli cells via the generation of reactive oxygen species and activation of the ERK signaling pathway. J Appl Toxicol. 2014;34(6): 628–636.
Casati L, Sendra R, Poletti A, Negri-Cesi P, Celotti F. Androgen receptor activation by polychlorinated biphenyls: epigenetic effects mediated by the histone demethylase Jarid1b. Epigenetics. 2013;8(10):1061–1068.
Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatr Res. 2007;61(5 pt 2):24R-29R.
Kafri T, Gao X, Razin A. Mechanistic aspects of genome-wide demethylation in the preimplantation mouse embryo. Proc Natl Acad Sci U S A. 1993;90(22):10558–10562.
Belzil VV, Bauer PO, Gendron TF, Murray ME, Dickson D, Petrucelli L. Characterization of DNA hypermethylation in the cerebellum of c9FTD/ALS patients. Brain Res. 2014;1584:15–21.
Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093.
Lechner M, Boshoff C, Beck S. Cancer epigenome. Adv Genet. 2010;70:247–276.
Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129(8):1983–1993.
Ng HH, Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev. 1999;9(2):158–163.
Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod Toxicol. 2008; 25(4):447–454.
Murugesan P, Senthilkumar J, Balasubramanian K, Aruldhas MM, Arunakaran J. Impact of polychlorinated biphenyl Aroclor 1254 on testicular antioxidant system in adult rats. Hum Exp Toxicol. 2005;24(2):61–66.
Colciago A, Casati L, Mornati O, et al. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat. Part 2: effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol. 2009;239(1):46–54.
Jacobson JL, Jacobson SW. Developmental Effects of PCBs in the Fish Eater Cohort Studies. PCBs—Recent Advances in the Environmental Toxicology and Health Effects of PCBs. Lexington, KY: The University Press of Kentucky;2001:127–136.
Odland J, Deutch B, Hansen JC, Burkow IC. The importance of diet on exposure to and effect of persistent organic pollutants on human health in the Arctic. Acta Paediatr. 2003;92(11): 1255–1266.
Sathish Kumar T, Sugantha Priya E, Raja Singh P, Arunakaran J. Lactational exposure of polychlorinated biphenyls downregulates critical genes in Leydig cells of F1 male progeny (PND21) [Published online October 26, 2016]. Andrologia. doi:10.1111/and. 12734.
Hertz-Picciotto I, Jusko TA, Willman EJ, et al. A cohort study of in utero polychlorinated biphenyl (PCB) exposures in relation to secondary sex ratio. Environ Health. 2008;7(1):1–8.
Buck GM, Vena JE, Schisterman EF, et al. Parental consumption of contaminated sport fish from Lake Ontario and predicted fecundability. Epidemiology. 2000;11(4):388–393.
Wu JP, Luo XJ, Zhang Y, et al. Bioaccumulation of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in wild aquatic species from an electronic waste (e-waste) recycling site in South China. Environ Int. 2008; 34(8):1109–1113.
Arnold DL, Mes J, Bryce F, et al. A pilot study on the effects of Aroclor 1254 ingestion by rhesus and cynomolgus monkeys as a model for human ingestion of PCBs. Food Chem Toxicol. 1990; 28(12):847–857.
Tabb MM, Blumberg B. New modes of action for endocrine disrupting chemicals. Mol Endocrinol. 2006;20(3):475–482.
Desaulniers D, Xiao GH, Lian H, et al. Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats. Int J Toxicol. 2009;28(4):294–307.
Sugantha Priya E, Sathish Kumar T, Balaji S, et al. Lactational exposure effect of polychlorinated biphenyl on rat Sertoli cell markers and functional regulators in prepuberal and puberal F1 offspring. J Endocrinol Invest. 2016;40(1);91–100.
Majumdar SS, Tsuruta J, Griswold MD, Bartke A. Isolation and culture of Sertoli cells from the testes of adult Siberian hamsters: analysis of proteins synthesized and secreted by Sertoli cells cultured from hamsters raised in a long or a short photoperiod. Biol Reprod. 1995;52(3):658–666.
Raychoudhury S, Thompson EW, Blackshaw A, Irving MG. Sertoli cells as paracrine modulators of DNA synthesis in rat peritubular myoid cells in culture. J Reprod Fertil. 1993;99(2): 513–518.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265–275.
Krishnamoorthy G, Selvakumar K, Venkataraman P, Elumalai P, Arunakaran J. Lycopene supplementation prevents reactive oxygen species mediated apoptosis in Sertoli cells of adult albino rats exposed to polychlorinated biphenyls. Interdiscip Toxicol. 2013; 6(2):83–92.
Skinner MK, Schlitz SM, Anthony CT. Regulation of Sertoli cell differentiated function: testicular transferrin and androgenbinding protein expression. Endocrinology. 1989;124(6): 3015–3024.
Jones PL, Veenstra GJ, Wade PA, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19(2):187–191.
Heckert LL, Daggett MA, Chen J. Multiple promoter elements contribute to activity of the follicle-stimulating hormone receptor (FSHR) gene in testicular Sertoli cells. Mol Endocrinol. 1998; 12(10):1499–1512.
Heckert LL. Activation of the rat follicle-stimulating hormone receptor promoter by steroidogenic factor 1 is blocked by protein kinase a and requires upstream stimulatory factor binding to a proximal E box element. Mol Endocrinol. 2001;15(5): 704–715.
Griswold MD, Kim JS. Site-specific methylation of the promoter alters deoxyribonucleic acid-protein interactions and prevents follicle-stimulating hormone receptor gene transcription. Biol Reprod. 2001;64(2):602–610.
Wu S, Zhu J, Li Y, et al. Dynamic epigenetic changes involved in testicular toxicity induced by di-2-(ethylhexyl) phthalate in mice. Basic Clin Pharmacol Toxicol. 2010;106(2):118–123.
Sekaran S, Jagadeesan A. In utero exposure to phthalate downregulates critical genes in Leydig cells of F1 male progeny. J Cell Biochem. 2015;116(7):1466–1477.
Chaudhary J, Skinner MK. E-box and cyclic adenosine monophosphate response elements are both required for folliclestimulating hormone-induced transferrin promoter activation in Sertoli cells. Endocrinology. 1999;140(3):1262–1271.
Regadera J, Martinez-Garcia F, Gonzalez-Peramatao P, Serrano A, Nistal M, Suárez-Quian C. Androgen receptor expression in Sertoli cells as a function of seminiferous tubule maturation in the human cryptorchid testis. J Clin Endocrinol Metab. 2001;86(1):413–421.
Karin M, Liu Z, Zandi E. AP1 function and regulation. Curr Opin Cell Biol. 1997:9(2):240–246.
O’Shea EK, Rutkowski R, Kim PS. Mechanism of specificity in the Fos-jun oncoprotein heterodimer. Cell. 1992;68(4):699–708.
Wise SC, Burmeister L A, Zhou XF, et al. Identification of domains of c-Jun mediating androgen receptor transactivation. Oncogene. 1998;16(15):2001–2010.
Bubulya A, Chen SY, Fisher CJ, Zheng Z, Shen XQ, Shemshedini L. c-Jun potentiates the functional interaction between the amino and carboxyl termini of the androgen receptor. J Biol Chem. 2001; 276(48):44704–44711.
Gaughan L, Logan IR, Cook S, Neal DE, Robson CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277(29):25904–25913.
Shang YF, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9(3):601–610.
Casati L, Sendra R, Sibilia V, et al. Endocrine disrupters: the new players able to affect the epigenome. Front Cell Dev Biol. 2015;3:37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugantha Priya, E., Sathish Kumar, T., Raja Singh, P. et al. Impact of Lactational Exposure to Polychlorinated Biphenyl Causes Epigenetic Modification and Impairs Sertoli Cells Functional Regulators in F1 Progeny. Reprod. Sci. 25, 818–829 (2018). https://doi.org/10.1177/1933719117699707
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117699707