Abstract
Objective
Our objective was to determine differences in properties of vaginal epithelial cells and the composition of vaginal secretions when Lactobacillus crispatus or Lactobacillus iners are numerically dominant in the vaginal microenvironment of pregnant women.
Methods
The vaginal microbiomes of 157 first-trimester pregnant women were identified by classifying partial 16S gene sequences amplified from the V1 to V3 region of bacterial ribosomal 16S RNA genes. The extent of autophagy and cell stress in vaginal epithelial cells was determined by measuring the intracellular levels of p62 and the inducible 70-kDa heat shock protein (hsp70). Vaginal secretions were analyzed using a colorimetric assay for D- and L-lactic acid and by enzyme-linked immunosorbent assay for matrix metalloproteinase 8, neutrophil gelatinase-associated lipocalin, α-amylase, hyaluronan, calprotectin, S100A8, and extracellular matrix metalloproteinase inducer (EMMPRIN).
Results
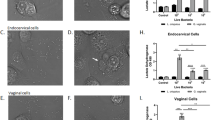
L. crispatus was dominant in 69 (43.9%) women, while L iners dominated in 23 (14.6%) women. The median epithelial p62 levels were 0.41 and 4.26 ng/mL in women with L crispatus or L iners dominance, respectively (P =.0035). The corresponding median hsp70 levels were 4.24 and 14.50 ng/mL, respectively (P <.0001). The D-lactic acid concentration in vaginal fluid was highest in association with L crispatus dominance, while all other vaginal fluid compounds except for EMMPRIN were highest when L iners was dominant (P <.03).
Conclusion
Epithelial cells exhibit a higher level of autophagy, lower induction of stress-related hsp70, and release lower level of mediators when L crispatus is most abundant as compared to when L iners dominates the vaginal microbiota.
Similar content being viewed by others
References
Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1(2):121–133.
Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive age women. Proc Natl Acad Sci U S A. 2011;108(suppl 1): 4680–4687.
Gajer P, Brotman RM, Gyoyun R, et al. Temporal dynamics of the human vaginal microbiota. Sci Trans Med. 2012;4(132): 132ra52.
van de Wijgert JHHM, Borgdorff H, Verhelst R, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9(8):e105998.
France MT, Mendes-Soares H, Forney LJ. Genomic comparisons of Lactobacillus crispatus and Lactobacillus iners reveal potential ecological drivers of community composition in the vagina. Appl Environ Microbiol. 2016;82(24):7063–7073. doi:10.1128/AEM.02385-16.
Macklaim JM, Gloor GB, Anukam KC, Cribby S, Reid G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc Natl Acad Sci U S A. 2011;108(suppl 1):4688–4695.
Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol. 2014;196(7):1458–1470.
Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818.
Maclaim JM, Fernandes AD, Di Bella JM, Hammond JA, Reid G, Gloor GB. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome. 2013;1(1);12–23.
Borgdorff H, Armstrong SD, Tytgat HLP, et al. Unique insights in the cervicovaginal Lactobacillus iners and L. crispatus proteomes and their association with microbiota dysbiosis. PLoS One. 2015; 11(3):e0150767.
Petricevic L, Domig KJ, Nierscher FJ, et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep. 2014;4:5136. doi:10.1038/srep05136.
Verstraelen H, Verheist R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal mcroflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol. 2009;9:116. doi:10.1186/1471-2180.9-116.
Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: friend or foe? [Published online November 30, 2016] Trends Microbiol. 2016;doi.org/10.1016/j.tm2016.11.007.
Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335.
Craig EA. The stress response: changes in eukaryotic gene expression in response to environmental stress. Science. 1985; 230(4727):800–801.
Radons J. The human hsp70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016;21(3):379–404.
Dokladny K, Zuhl MN, Mandell M, et al. Regulatory coordination between two major intracellular homeostatic systems. Heat shock response and autophagy. J Biol Chem. 2013;288(21):14959–14972.
Dokladny K, Myers OB, Moseley PL. Heat shock response and autophagy—cooperation and control. Autophagy. 2015;11(2): 200–213.
Kanninen TT, Sisti G, Witkin SS. Induction of the 70 kDa heat shock protein stress response inhibits autophagy: possible consequences for pregnancy outcome. J Matern Fetal Neonatal Med. 2016;29(1):159–162.
Shen J, Song N, Williams CJ, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep. 2016;6:24380.
Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ. Evaluation of a method for the extraction and purification of DNA from the human microbiome. PLoS One. 2012;11(9):e0161148.
Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: Implications for protection against upper genital tract infections. MBio. 2013;4(4):e00460-13.
Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a speciesspecific manner. J Infect Dis. 2014;209(12):1989–1999.
Rizzo A, Losacco A, Carratelli CR. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human b-defensins 2 and 3. Immunol Lett. 2013;156(1-2):102–109.
Abdel-Nour M, Tsalikis J, Kleinman D, Ginardin SE. The emerging role of mTOR signaling in antibacterial immunity. Immunol Cell Biol. 2014;92(4):346–353.
Feng Y, Yao Z, Klionsky DJ. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015;25(6):354–363.
Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5): 307–318.
Davies EL, Bacelar FVG, Marshall MJ, et al. Heat shock proteins form part of a danger signal cascade in response to lipopolysaccharide and GroEL. Clin Exp Immunol. 2006;145(1):183–189.
Beghini J, Giraldo PC, Linhares IM, Ledger WJ, Witkin SS. Neurophil gelatinase-associated lipocalin concentration in vaginal fluid: relation to bacterial vaginosis and vulvovaginal candidiasis. Reprod Sci. 2015;22(8):964–968.
Nasioudis D, Witkin SS. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med Microbiol Immunol. 2015;204(4):471–479.
Abtin A, Eckhart L, Gla¨ser R, Gmeiner R, Mildner M, Tschachler E. The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes. J Invest Dermatol. 2010;130(10):2423–2430.
Diaz-Ochoa VE, Jellbauer S, Klaus S, Raffatellu M. Transition metal ions at the crossroads of mucosal immunity and microbial pathogenesis. Front Cell Infect Microbiol. 2014;4:2.
Rahkonen L, Rutanen EM, Unkila-Kallio L, et al. Factors affecting matrix metalloproteinase-8 levels in the vaginal and cervical fluids in the first and second trimester of pregnancy. Hum Reprod. 2009;24(11):2693–2702.
Turley EA, Noble PW, Bourguignon LYW. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–4592.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leizer, J., Nasioudis, D., Forney, L.J. et al. Properties of Epithelial Cells and Vaginal Secretions in Pregnant Women When Lactobacillus crispatus or Lactobacillus iners Dominate the Vaginal Microbiome. Reprod. Sci. 25, 854–860 (2018). https://doi.org/10.1177/1933719117698583
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117698583