Abstract
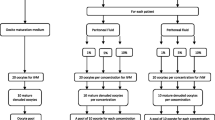
Some studies have demonstrated alterations in the composition of peritoneal fluid (PF) from women with endometriosis. Controversial studies have suggested that impaired oocyte quality may be involved in the pathogenesis of endometriosis-related infertility. The aim of this study was to evaluate the spindle and chromosome distribution of in vitro-matured oocytes in the presence of 2 concentrations of PF from infertile women with minimal/mild endometriosis (EI/II) compared to fertile controls. We performed an experimental study using a bovine model. Samples of PF were obtained from 12 women who underwent video-laparoscopy—6 infertile women with EI/II and 6 fertile women without endometriosis (control group). Immature bovine oocytes underwent in vitro maturation (IVM) in the absence of PF and in the presence of 2 concentrations (1% and 10%) of PF from fertile women and from infertile women with EI/II. After 22 to 24 hours of IVM, oocytes were fixed for subsequent immunofluorescence staining for the visualization of microtubules and chromosomes by confocal microscopy. The percentage of meiotically normal oocytes was significantly lower for oocytes that underwent IVM in the presence of 1% (62.50%) and 10% (56.25%) of PF from infertile women with EI/II than in the absence of PF (88.46%) and in the presence of 1% (78.57%) and 10% (84.61%) of PF from fertile women (P <.01). We demonstrated that PF from infertile women with EI/II promotes meiotic abnormalities in in vitro- matured bovine oocytes. Therefore, our results contribute to the understanding of the etiopathogenic mechanisms of infertility related to EI/II.
Similar content being viewed by others
References
Minici F, Tiberi F, Tropea A, et al. Endometriosis and human infertility: a new investigation into the role of eutopic endometrium. Hum Reprod. 2008;23(3):530–537.
Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13(1):126–134.
Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441–447.
Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstetr Gynecol Clin North Am. 2012;39(4):535–549.
Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90(2):247–257.
Carvalho LFP, Below A, Abrão MS, Agarwal A. Minimal and mild endometriosis negatively impact on pregnancy outcome. Rev Assoc Méd Bras (1992). 2012;58(5):607–614.
Marcoux S, Maheux R. Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337(4):217–222.
Bérubé S, Marcoux S, Langevin M, Maheux R. Fecundity of infertile women with minimal or mild endometriosis and women with unexplained infertility. The Canadian Collaborative Group on Endometriosis. Fertil Steril. 1998;69(6):1034–1041.
Akande VA, Hunt LP, Cahill DJ, Jenkins JM. Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Hum Reprod. 2004;19(1):96–103.
Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometrio-sis on in vitro fertilization. Fertil Steril. 2002;77(6):1148–1155.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799.
Simón C, Gutierrez A, Vidal A, et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9(4):725–729.
Garrido N, Navarro J, Remohi J, Simon C, Pellicer A. Follicular hormonal environment and embryo quality in women with endo-metriosis. Hum Reprod Update. 2000;6(1):67–74.
Pellicer A, Navarro J, Bosch E, et al. Endometrial quality in infertile women with endometriosis. Ann N Y Acad Sci. 2001; 943:122–130.
Ferreira EM, Vireque AA, Adona PR, Ferriani RA, Navarro PA. Prematuration of bovine oocytes with butyrolactone I reversibly arrests meiosis without increasing meiotic abnormalities after in vitro maturation. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):76–80.
Barcelos ID, Vieira RC, Ferreira EM, et al. Anomalias meióticas de oócitos de pacientes com endometriose submetidas à estimu-lação ovariana [in Portuguese]. Rev Bras Ginecol Obstet. 2008; 30(8):413–419.
Barcelos ID, Vieira RC, Ferreira EM, Martins WP, Ferriani RA, Navarro PA. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with endometriosis and from control subjects: a pilot study. Fertility and Sterility. 2009;92(5):1749–1752.
Hu Y, Betzendahl I, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Hum Reprod. 2001;16(4):737–748.
Eichenlaub-Ritter U, Shen Y, Tinneberg HR. Manipulation of the oocyte: possible damage to the spindle apparatus. Reprod Biomed Online. 2002;5(2):117- 124.
Mullen SF, Agca Y, Broermann DC, Jenkins CL, Johnson CA, Critser JK. The effect of osmotic stress on the metaphase II spindle of human oocytes, and the relevance to cryopreservation. Hum Reprod. 2004;19(5):1148–1154.
Navarro PA, Liu L, Keefe DL. In vivo effects of arsenite on meiosis, preimplantation development, and apoptosis in the mouse. Biol Reprod. 2004;70(4):980–985.
Navarro PA, Liu L, Ferriani RA, Keefe DL. Arsenite induces aberrations in meiosis that can be prevented by coadministration of N-acetylcysteine in mice. Fertil Steril. 2006;85(1):1187–1194.
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–843.
Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79(6):1288–1293.
Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysopho-sphatidylcholine, a chemotactic factor for monocytes/ T-lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab. 1998;83(6):2110–2113.
Bedaiwy MA, Falcone T. Peritoneal fluid environment in endometriosis. Clinicopathological implications. Minerva Ginecol. 2003;55(4):333–345.
Ma CH, Yan LY, Qiao J, et al. Effects of tumor necrosis factor-alpha on porcine oocyte meiosis progression, spindle organization, and chromosome alignment. Fertil Steril. 2010;93(3):920–926.
Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821.
Cooper TG, Noonan E, Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245.
Adona PR, Lima Verd, Leal C. Meiotic inhibition with different cyclin-dependent kinase inhibitors in bovine oocytes and its effects on maturation and embryo development. Zygote. 2004; 12(3):197–204.
Hashimoto S, Minami N, Takakura R, Imai H. Bovine immature oocytes acquire developmental competence during meiotic arrest in vitro. Biol Reprod. 2002;66(6):1696–1701.
Liu L, Ju JC, Yang X. Differential inactivation of maturation-promoting factor and mitogen-activated protein kinase following parthenogenetic activation of bovine oocytes. Biol Reprod. 1998; 59(3):537–545.
Ju JC, Jiang S, Tseng JK, Parks JE, Yang X. Heat shock reduces developmental competence and alters spindle configuration of bovine oocytes. Theriogenology. 2005;64(8):1677–1689.
Liu F, He L, Liu Y, Shi Y, Du H. The expression and role of oxidative stress markers in the serum and follicular fluid of patients with endometriosis. Clin Exp Obstetr Gynecol. 2013; 40(3):372–376.
Da Broi MG, Malvezzi H, Paz CC, Ferriani RA, Navarro PA. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovin, metaphase II oocytes. Hum Reprod. 2014;29(2):315–323.
Giorgi VSI, Da Broi MG, Paz CCP, Ferriani RA, Navarro PA. N-acetyl-cysteine and L-carnitine prevent meiotic oocyte damage induced by follicular fluid from infertile women with endome-triosis. Reprod Sci. 2016;23(3):342–351.
Li Y, Feng HL, Cao YJ, et al. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85(4):827–832.
Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14(4):345–357.
Mansour G, Sharma RK, Agarwal A, Falcone T. Endometriosis-induced alterations in mous, metaphase II oocyte microtubules and chromosomal alignment: a possible cause of infertility. Fertil Steril. 2010;94(5):1894–1899.
Augoulea A, Alexandrou A, Creatsa M, Vrachnis N. Lambrinou-daki I. Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress. Arch Gynecol Obstetr. 2012;286(1):99–103.
Santulli P, Chouzenoux S, Fiorese M, et al. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum Reprod. 2015;30(1):49–60.
Mier-Cabrera J, Jiménez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernández-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118(1):6–16.
Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653.
Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000;63(3):805–810.
Shaeib F, Khan SN, Ali I, et al. The defensive role of cumulus cells against oxygen species insult in metaphase II mouse oocytes. Reprod Sci. 2016;23(4):498–507.
Mansour G, Abdelrazik H, Sharma RK, Radwan E, Falcone T, Agarwal A. L-carnitine supplementation reduces oocyte cytoske-leton damage and embryo apoptosis induced by incubation in peritoneal fluid from patients with endometriosis. Fertil Steril. 2009;91(5):2079–2086.
Malhi PS, Adams GP, Singh J. Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics. Biol Reprod. 2005;73(1):45–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jianini, B.T.G.M., Giorgi, V.S.I., Da Broi, M.G. et al. Peritoneal Fluid From Infertile Women With Minimal/Mild Endometriosis Compromises the Meiotic Spindle of Metaphase II Bovine Oocytes: A Pilot Study. Reprod. Sci. 24, 1304–1311 (2017). https://doi.org/10.1177/1933719116687658
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116687658