Abstract
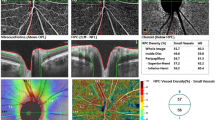
This study aimed to investigate the macular, retinal nerve fiber layer (RNFL), and choroid thickness alterations using spectral-domain optical coherence tomography in women with polycystic ovary syndrome (PCOS) and to compare them with healthy reproductive-age women volunteers. Study group consisted of 64 patients with PCOS and control group consisted of 60 healthy volunteers. There was a statistically significant difference between PCOS and control groups for choroid thickness (P < .001). Fovea center thickness and temporal inner macula were significantly thinner in the PCOS group than those in the healthy control group (P = .009 and P = .033, respectively). Contrary to these findings, nasal outer macula (NOM) and temporal outer macula (TOM) were statistically thicker in the PCOS group than those in the control group (P = .001 and P < .001, respectively). Increased choroid thickness and RNFL may lead to increase in both retinal volume and retinal thickness in the peripheral side of the retina. Therefore, NOM and TOM region can be accepted sensitive areas in patients with PCOS.
Similar content being viewed by others
References
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Frank S. Polycystic ovary syndrome. N Engl J Med. 1995; 333(13):853–861.
Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012; 33(5):812–841.
Escobedo LG, Lee NC, Peterson HB, Wingo PA. Infertility associated endometrial cancer risk may be limited to specific subgroups of infertile women. Obstet Gynecol. 1991;77(1):124–128.
Açmaz G, Albayrak E, Acmaz B, et al. Level of anxiety, depression, self esteem, social anxiety and quality of life among the women with polycystic ovary syndrome. Scientific WorldJournal. 2013;2013:851815. doi: 10.1155/2013/851815.
Fuchsjager-Mayrl G, Nepp J, Schneeberger C, et al. Identification of estrogen and progesterone receptor mRNA expression in the conjunctiva of premenopausal women. Invest Ophthalmol Vis Sci. 2002;43(9):2841–2844.
Vecsei PV, Kircher K, Kaminski S, Nagel G, Breitenecker G, Kohlberger PD. Immunohistochemical detection of estrogen and progesterone receptor in human cornea. Maturitas. 2000;36(3): 169–172.
Esmaeli B, Harvey JT, Hewlett B. Immunohistochemical evidence for estrogen receptors in meibomian glands. Ophthalmology. 2000;107(1):180–184.
Early Treatment Diabetic Retinopathy Study Research Group. ETDRS Report No. 7: Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. Ophthalmology. 1991;98(5 suppl):741–756.
March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551.
Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. 2010;95(5): 2038–2049.
Kiddy DS, Sharp PS, White DM, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf). 1990;32(2):213–220.
Sommers SC, Hertig AT, Bengloff H. Genesis of endometrial carcinoma. II. Cases 19 to 35 years old. Cancer. 1949;2(6):957–963.
Shu XO, Brinton LA, Zheng W, Gao YT, Fan J, Fraumeni-JF Jr. A population-based case-control study of endometrial cancer in Shanghai, China. Int J Cancer. 1991;49(1):38–43.
Modan B, Ron E, Lerner GL, et al. Cancer incidence in a cohort of infertile women. Am J Epidemiol. 1998;147(11):1038–1042.
Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb). 2000;3(2):101–105.
Siesky BA, Harris A, Patel C, et al. Comparison of visual function and ocular hemodynamics between pre- and post-menopausal women. Eur J Ophthalmol. 2008;18(2):320–323.
Lang Y, Lang N, Ben-Ami M, Garzozi H. The effects of hormone replacement therapy (HRT) on the human eye. Harefuah. 2002; 141(3):287–291.
Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181.
Menke MN, Dabov S, Knecht P, Sturm V. Reproducibility of retinal thickness measurements in healthy subjects using spectralis optical coherence tomography. Am J Ophthalmol. 2009;147(3): 467–472.
Curcio CA, Allen KA, Sloan KR, et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol. 1991;312(4):610–624.
Ciccone MM, Cicinelli E, Giovanni A, et al. Ophthalmic artery vasodilation after intranasal estradiol use in postmenopausal women. J Atheroscler Thromb. 2012;19(12):1061–1065.
Atalay E, Karaali K, Akar M, et al. Early impact of hormone replacement therapy on vascular hemodynamics detected via ocular colour Doppler analysis. Maturitas. 2005;50(4):282–288.
Sogawa K, Nagaoka T, Takahashi A, et al. Relationship between choroidal thickness and choroidal circulation in healthy young subjects. Am J Ophthalmol. 2012;153(6):1129–1132.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Açmaz, G., Ataş, M., Gülhan, A. et al. Evaluation of the Macula, Retinal Nerve Fiber Layer, and Choroid Thickness in Women With Polycystic Ovary Syndrome Using Spectral-Domain Optical Coherence Tomography. Reprod. Sci. 21, 1044–1049 (2014). https://doi.org/10.1177/1933719114522523
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719114522523