Abstract
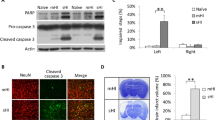
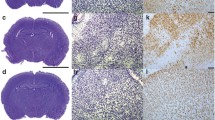
In utero exposure to infection or inflammation is a strong and independent predictor of cerebral palsy. Using a rat model of neonatal hypoxic—ischemic (HI) encephalopathy, we investigated the hypothesis that C-reactive protein (CRP), which is not specific for infection, aggravates vulnerability of the immature brain to HI. Seven-day-old rats were divided into human CRP treated and control groups. After injection of each solution, they underwent left common carotid artery ligation and exposure to 8% hypoxia for 40 minutes. Human CRP, rat CRP, and interleukin 6 (IL-6) concentrations in serum were measured by enzyme-linked immunosorbent assay 30 to 60 minutes after injection of each solution. Four days later, microtubule-associated protein 2 (MAP-2) immunostaining was used to examine the brains for neuronal damage. Human CRP treatment significantly reduced the MAP-2 positive area ratio, compared with control group (P < .05), suggesting that human CRP-enhanced susceptibility to HI-induced brain damage. Mean serum human CRP concentration in the human CRP group was 1823 ± 520 ng/mL (range: 365–3964 ng/mL). Interleukin 6 concentrations in serum were moderately elevated in both groups, without significant differences, and rat CRP concentrations were within normal range. C-reactive protein makes the immature brain susceptible to HI insult, even if the insult causes little or no injury by itself.
Similar content being viewed by others
References
Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. Multivariate analysis of risk. N Engl J Med. 1986;315(2):81–86.
Graham EM, Petersen SM, Christo DK, Fox HE. Intrapartum electronic fetal heart rate monitoring and the prevention of perinatal brain injury. Obstet Gynecol. 2006;108(3 pt 1): 656–666.
Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278(3):207–211.
Murphy DJ, Sellers S, MacKenzie IZ, Yudkin PL, Johnson AM. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet. 1995;346(8988):1449–1454.
Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11): 1417–1424.
Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998 Jul;179(1):194–202.
Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11(1):18–25.
Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42(1):1–8.
Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000; 182(3):675–681.
Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174(5):1433–1440.
Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003; 14(2):85–90.
Ziakas A, Gavrilidis S, Giannoglou G, et al. In-hospital and long-term prognostic value of fibrinogen, CRP, and IL-6 levels in patients with acute myocardial infarction treated with thrombolysis. Angiology. 2006;57(3):283–293.
Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001;32(4):917–24.
Suleiman M, Khatib R, Agmon Y, et al. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol. 2006;47(5):962968.
Bursi F, Weston SA, Killian JM, Gabriel SE, Jacobsen SJ, Roger VL. C-reactive protein and heart failure after myocardial infarction in the community. Am J Med. 2007;120(7): 616–622.
Muir KW, Weir CJ, Alwan W, Squire IB, Lees KR. C-reactive protein and outcome after ischemic stroke. Stroke. 1999 May;30(5):981–5.
Lagrand WK, Niessen HW, Wolbink GJ, et al. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95(1): 97–103.
Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440(7088):1217–1221.
Nijmeijer R, Lagrand WK, Lubbers YT, et al. C-reactive protein activates complement in infarcted human myocardium. Am J Pathol. 2003;163(1):269–275.
Griselli M, Herbert J, Hutchinson WL, et al. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190(12): 1733–1740.
Gill R, Kemp JA, Sabin C, Pepys MB. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J Cereb Blood Flow Metab. 2004;24(11):1214–1218.
De Beer FC, Pepys MB. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31.
Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141.
Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997 Jul;422:85–8.
Kitagawa K, Matsumoto M, Niinobe M, et al. Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage—immunohistochemical investigation of dendritic damage. Neuroscience. 1989;31(2):401–411.
Tomimatsu T, Fukuda H, Kanagawa T, Mu J, Kanzaki T, Murata Y. Effects of hyperthermia on hypoxic-ischemic brain damage in the immature rat: its influence on caspase-3-like protease. Am J Obstet Gynecol. 2003;188(3):768–773.
Fukuda H, Tomimatsu T, Kanagawa T, et al. Postischemic hyperthermia induced caspase-3 activation in the newborn rat brain after hypoxia-ischemia and exacerbated the brain damage. Biol Neonate. 2003;84(2):164–171.
Fukui O, Kinugasa Y, Fukuda A, et al. Post-ischemic hypothermia reduced IL-18 expression and suppressed microglial activation in the immature brain. Brain Res. 2006; 1121(1):35–45.
Vannucci RC, Lyons DT, Vasta F. Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke. 1988;19(2):245–250.
Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011(1): 48–57.
Dammann O, Leviton A. Brain damage in preterm newborns: biological response modification as a strategy to reduce disabilities. J Pediatr. 2000;136(4):433–438.
Yoon BH, Romero R, Kim CJ, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177(2): 406–411.
Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007; 197(3):294 e1–e6.
Pepys MB, Hawkins PN, Kahan MC, et al. Proinflammatory effects of bacterial recombinant human C-reactive protein are caused by contamination with bacterial products, not by C-reactive protein itself. Circ Res. 2005;97(11):e97–e103.
Clapp BR, Hirschfield GM, Storry C, et al. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111(12):1530–1536.
Sjoberg AP, Trouw LA, McGrath FD, Hack CE, Blom AM. Regulation of complement activation by C-reactive protein: targeting of the inhibitory activity of C4b-binding protein. J Immunol. 2006;176(12):7612–620.
Zhang J, Rui YC, Yang PY, Lu L, Li TJ. C-reactive protein induced expression of adhesion molecules in cultured cerebral microvascular endothelial cells. Life Sci. 2006;78(26): 2983–2988.
Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165–2168.
Qamirani E, Ren Y, Kuo L, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25(5):995–1001.
Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–919.
Cowell RM, Plane JM, Silverstein FS. Complement activation contributes to hypoxic-ischemic brain injury in neonatal rats. J Neurosci. 2003;23(28):9459–9468.
Maroko PR, Carpenter CB, Chiariello M, et al. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978;61(3):661–670.
Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003; 110(suppl 20):124–127.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6): 448–454.
Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22(2):455–463.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kinugasa-Taniguchi, Y., Tomimatsu, T., Mimura, K. et al. Human C-Reactive Protein Enhances Vulnerability of Immature Rats to Hypoxic—Ischemic Brain Damage: A Preliminary Study. Reprod. Sci. 17, 419–425 (2010). https://doi.org/10.1177/1933719110361379
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719110361379