Abstract
Radionuclides are widely known to produce serious problem when released and dispersed in the environment because they can contaminate humans through food chains, affecting metabolic process and causing health diseases to the population. A large amount of radionuclides is produced as waste during the process of nuclear facility operation, maintenance, and decommissioning. Wastewater contains many radioactivemetal ions. During the last three decades, after Chernobyl accident and then that of Fukushima, several techniques have been developed for the removal of radionuclides from the environment and from wastewater aiming of inertization. One of the most recent techniques, and in continuous progress, is the radionuclides removal by adsorption using natural and/or synthesized materials having high retention capacity and being resistant to radiation. The most used type of material, cheap and easy-to-find, is zeolite due to its high ion exchange capacity, adsorption efficiency and abundance. This work concerns a preliminary study on zeolites identification for the removal of radionuclides in wastewater samples from the fuel storage of the former nuclear power plant of Latina, Italy. The activity concentration of the radionuclides in the samples was investigated by γ- and α-spectrometry. The wastewater samples contain the radionuclides 137Cs, 60Co and 241Am, and the best solution for their removal was discovered to be the zeolite A. The obtained results can be enlarged to all former Italian nuclear facilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, with the global energy consumption increasing, scientific attention was focused on the development of economic and clean energy sources. Nuclear power provides economical production of electricity without emitting greenhouse gases, being an alternative to thermal power derived from fossil fuels [1]. On the other hand, however, nuclear industry generates a large amount of radioactive waste in the utilization of nuclear energy, which requires rigorous downstream treatments [2]. Radioactive wastes consist of materials, containing unstable atoms that emit ionizing radiation as they decay (radionuclides), which are no longer useful and must be kept isolated from the environment if the levels of radioactivity (activity concentrations of the radionuclides) are considered harmful [3]. Radionuclides are transmitted to humans through the food chains, affecting the metabolic process, and can cause health diseases in the population. Radionuclides from nuclear facilities can be: fission products (e.g., 137Cs, 90Sr and 134Cs), activation products (e.g., 60Co, 58Co, 54Mn and 65Zn), and transuranic (e.g., 239Pu and 241Am). All of them exhibit long half-lives and a high water solubility that causes them to rapidly spread to the environment [4]. Contaminated wastewater is aqueous waste usually containing high concentrations of radionuclides released during routine operations at nuclear facility: laundry, decontamination, and decommissioning of wastewater [5]. Radioactive wastewater generated from nuclear power plants should be safely managed because unintended release can lead to serious contamination of the natural environment and threats to human health [6]. The safe discharge of such liquid radioactive waste into the environment has to fulfill very strict requirements: the waste must be post-treated to reduce the volume and the concentration of radioactive elements, so as to meet international and national regulations [7]. Hence, the urgent need to address the safety problems caused by radioactive wastewater is a current issue. There are several methods used to treat radioactive wastewater to have a considerable reduction in the volume of radiologically contaminated aqueous solutions and a significant decontamination process: flocculation, precipitation, membrane separation, evaporation, ion exchange and adsorption [8]. The latter two have received increasing attention as they require less use of organic solvents, thus making the process simpler and safer. The materials most used in decontamination treatments include zeolite, vermiculite, vanadosilicates, silicotitanates, metal sulfides, crystalline silicotitanate and metal hexacyanoferrates [9]. Among them, zeolites have been reported to be excellent adsorbents for heavymetal removal from aqueous solutions by ion exchange [10]. The other above-reported inorganic materials show very low adsorption efficiency and/or require artificial synthesis. In addition, zeolites were extensively investigated as they can withstand the harsh conditions of nuclear sites, which produce immense heat and radiation. In this regard, various advantages of zeolites are: low price, abundant, commercial availability, large ion exchange capacity, excellent selectivity, thermal stability as well as antiradiation stability, diverse structures and compositions, and high compatibility with cement and glass matrices that are used for the immobilization of radioactive cations [11]. The mechanism of radionuclides removal is: the cations in zeolite structure can freely exchange with cations in the aqueous solutions through the cavities, thanks to zeolite network-like structure containing uniform small pores [12]. Hence, zeolites have drawn a great deal of interest, and still growing, among researchers and scientists worldwide due to their high advantages for radionuclide removal in liquid waste. Within this topic, the present work investigates the case study of radioactive wastewater from the former Latina nuclear power plant, in Italy. The γ- and α-spectrometric analysis has been performed on several samples, together with the study of best zeolites, for selected radionuclide removal, to be used in decontamination processes. The aim is to test and validate a standardized procedure that could be a treatment solution to be used in similar industries or that produce this kind of wastewater.
2 Materials and methods
2.1 The former nuclear power plant of Latina (Italy)
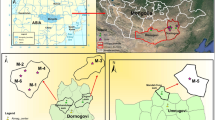
In Latina, a city in the Center Italy about 60 km from Rome, there is one of the four former nuclear power plants in Italy, started in 1963 and shotdown in 1987 (Fig. 1a and b). It was the first nuclear power plant built in Italy [13]. Two of the other three are located in Northern Italy nearby Milan, namely the Caorso plant started in 1978 and shotdown in 1990, and the Trino plant started in 1964 and shotdown in 1990 (Fig. 1a). Only the Garigliano plant, started in 1964 and shotdown in 1982, is located in Southern Italy nearby Naples (Fig. 1a). The facilities were closed after the referendum to abrogate the nuclear power supply in November 1987; except the Garigliano closed due to a breakdown. Since 1999 they are currently being decommissioned by the SOGIN company, under control of the Italian Ministry of Economy and Finance, responsible for the management of radioactive waste in Italy, including those produced by industrial, research and nuclear medicine activities (www.sogin.it). The former Latina nuclear power plant is equipped with a gas graphite reactor GCR-Magnox, with an electrical power of 200 MWe (Fig. 1c). The plant has operated regularly with an average availability factor of 76% and a maximum of 96%, producing a total of about 26 billion kWh. The decommissioning of the former plant is based on three macro-activities: (i) reduction and dismantling of the reactor building; (ii) treatment and storage of radioactive waste; (iii) auxiliary buildings dismantling (pond building, ‘mud pit’ building, effluent treatment building (radwaste), etc.) (Fig. 1c) [14]. All these points are designed to be in compliance with the following fundamental objectives: minimization of nuclear and conventional risk for the operators involved in carrying out the activities; minimization of the environmental impact; minimization and optimization of the quantities of radioactive waste produced, according to the final storage at the national repository; management of waste materials in accordance with the legislation (formerly D.lgs 230/95, currently D.lgs 101/20) [15]. The objectives are achieved through continuous and constant radiometric controls. The safe release of the site, without radiological risk for the individual, the community and the environment, is scheduled for 2042. According to Legislative Decree 15 February 2010 n. 31 (D.lgs 31/2010) [16], radioactive wastes can be stored in superficial disposal plants with simple barriers, or in superficial plants, or at shallow depths, with engineering barriers, or in plants inside geological formations, as their radioactivity levels increase.
2.2 Radioactive wastewater source
The investigated wastewater samples from the former nuclear power plant of Latina come from the ‘mud pit’ building (Fig. 1c). In particular, the aqueous waste is the supernatant, overlying the solid phase of the mud, that stratifies on the bottom, and separated from the liquid, in which they were mixed, by precipitation or sedimentation [14]. The ‘mud pit’ building is a prefabricated shed with a metal beam bearing structure and metal panel infill, composed of two parts: the mud pit itself, and the mud extraction plant. The mud pit consists of a tank without a cover, made of stainless steel with a diameter of 7.50 m and a height of 3.90 m placed in an underground rectangular structure of reinforced concrete, coated on the outside of the walls and the bottom with a liner of material synthetic and internally finished with high thickness decontaminable paint. The radioactive mud contained in the tank derives from various sources, mainly from the periodic cleaning of the pond building and from the drainage of the radwaste sedimentation treatment. The pond building is a reinforced concrete structure of 12 × 37 m2, located on the south side of the reactor building, between the liquid effluent treatment building (radwaste) and the ‘pit splitters’ building; inside there is the 1200 m2 pool for cooling and storing the irradiated fuel elements. The ‘pit splitters’ building, with the same construction characteristics of the ‘mud pit’ building and an area of 450 m2, has the main function to protect the previous two described buildings from bad weather, via two pit ventilation and filtration systems.
2.3 Zeolite materials
Zeolites are naturally occurring minerals group with a porous crystalline structure (Fig. 2a) making them considered “natural molecular sieves” [17].
They are a family of open-framework aluminosilicates with orderly distributed micropores, and the general chemical formula is Mx/n((AlO2)x(SiO2)y)(H2O)z; where M represents an extra-framework cation of valence n (alkali metal or alkaline earth metal cation), which compensates the negative charges of the framework and allows zeolite to be a cation exchangers; z is the amount of water of crystallization per unit cell; x and y represent the total number of the [SiO4]4− and [AlO4]5− tetrahedra in a unit cell of zeolite, respectively (Fig. 2b) [17]. Zeolite has three-dimensional structures composed of the networks of [SiO4]4− and [AlO4]5− tetrahedra linked to each other with oxygen atoms. The Si-AlO4 tetrahedra connected among them conform the framework; different connection ways lead to a diversity of zeolites and hundreds of zeolites are known [18]. The frameworks are formed by rings that correspond to pore opening windows of zeolites. Depending on the structural type, cages are formed by connecting the pore openings of defined sizes in the tetrahedral structures [4]. Zeolites can be also prepared in the laboratory. In fact, they are usually divided into two main categories: natural (e.g., clinoptilolite, mordenite and chabazite), and synthetic (e.g., A, X and Y). For instance, clinoptilolite, chabazite and zeolite A belong to the small/medium-pore (8/10-ring) zeolites; while mordenite, zeolites X and Y are large-pore (12-ring) zeolites. These material has many advantages, such as low price, large ion exchange capacity, excellent selectivity, thermal and radiation stability, high sorption capacity, and the ability to adjust the pH of the aqueous system [11]. Due to the regular crystalline porous structures and the molecular sieve property, nowdays zeolite is used in several application fields from research to medicine and industry: chemical sensors in industrial process control, environmental mitigation, gas capture, indoor air-quality monitoring, medical monitoring, catalysts of oil refineries, removing contaminants in laundry, catalysts in petrochemical industries, biomass conversion, fuel cells, thermal energy storage, CO2 capture and conversion, air-pollution remediation, control of N2O emissions, and water purification [19]. In the field of the nuclear fuel cycle, zeolites has been attempted to be applied as the cation exchange adsorbent for heavy metal ions from liquid solutions, e.g., for the sorption and removal of various hazardous species, including radionuclides, toxic metals and gases [20]. Cations in zeolite network-like structure exchange with cations in the waste aqueous solutions freely through the uniform small pores (0.3–1.0 nm) (Fig. 2c) [21]. The radiation resistance, high adsorption, exchange capacity and specific radionuclides affinity-selectivity of zeolites depend on the network-like structure and the silica-alumina ratio, as well as in the presence of alkali cations in zeolite framework. Examples have been the extensive use of clinoptilolite in dealing with the effects of Chernobyl accident, while chabazite in the Three Mile Island clean-up [22].
2.4 Spectrometric analysis on radioactive wastewater
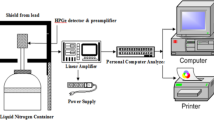
The characterization of the radioactivity content on the investigated radioactive wastewater samples, from the former Latina nuclear power plant, has been made by high resolution γ-ray spectrometry and α-spectrometry. The γ-spectrometry system consists of a coaxial high purity germanium (HPGe) detector, having efficiency of 40% and energy resolution of 1 keV at 122 keV and 1.9 keV at 1332 keV. The detector is equipped with liquid nitrogen (LN2) refrigeration system and set of lead collimators to shield against external background. Energy calibration and efficiency were obtained using appropiate multi-γ sources. The minimum detectable activity (MDA) values range between 1 and 10 Bq·L−1. The background spectrum was also collected and subtracted from wastewater sample spectrum. The samples were placed in Marinelli beakers of 500 ml. More details on γ-spectrometry are reported in [23]. The α-spectrometry was performed by a silicon solid-state detectors with very high efficiency for ultra-low background application. The samples were prepared with the procedure of evaporation onto thin layer of a stainless steel disk [3, 8]. The measurement was performed under vacuum condition to avoid α-particles energy loss. Energy calibration and efficiency were obtained using a multi-α sources. The MDA was 0.02 Bq·L−1. The measurement time was 2 h, to obtain a sufficiently high counting statistic. More details on α-spectrometry are reported in [24]. The acquisition and the analysis of spectra from γ- and α-spectrometry were carried out using the GammaVision and AlphaVision software, for the automatic calculation of the activity concentration associated with the material being measured. These tools are developed in such a way as to have a sufficient accuracy, taking into account the characteristics of the sample, the geometric characteristics of the sample container and its filling and the distance between the container and the detector. The activity concentrations uncertainties were calculated by considering the errors associated with counting and peaks analysis, emission probability, energy and efficiency calibration, and sample mass. The spectrometry systems occupy a fixed position, and maintain a constant measurement configuration; in particular, a template is preliminarily prepared for placing the container to be subjected to measurement, ensuring the correct radiometric control position with respect to the detector [14].
3 Results and discussion
The average of activity concentration (Bq·L−1) results from the γ-spectrometry on the aqueous (supernatant) samples of radioactive waste from the ‘mud pit’ building of the former Latina nuclear power plant are reported in Table 1. The main radionuclides of artificial origin, beyond the MDA levels, are 241Am, 137Cs and 60Co, generated during the process of nuclear facility operation. These radionuclides, with similar activity concentrations, can be found in wastewater from the former Italian nuclear facilities [14,15,16]. The full energy γ-peaks at 59.5 keV and 661.7 keV were used for the activity concentration determination of 241Am and 137Cs, respectively; while for 60Co the two γ-peaks at 1173.2 keV and 1332.5 keV were used. The results from the α-spectrometry on the wastewater samples is also reported in Table 1: the average of the gross α-activity concentration was computed, mostly due to the 241Am α-decay at 5485.56 keV energy. According to the current Italian regulation D.lgs 101/2020 [7], implementation of the European Directive 59/2013/EURATOM [15] concerning basic safety standards relating to protection against ionizing radiation, the limit of γ-activity concentration in wastewater samples for exemption/clearance is 100 Bq·L−1 for 241Am, 137Cs and 60Co for single radionuclide; the same value is adopted as average limit for gross α-activity concentration. For mixture of radionuclides, as in the wastewater samples, the D.lgs 101/2020 establishes that the sum of the ratios of the γ-activity concentration value of the single radionuclide and the correspondent limit value is less than 1. These values arise from various editions, occurred over the years, of recommendations of the International Commission on Radiological Protection (ICRP) and Radiation Protection manuals (RP). In Table 1, the only value of activity concentration of 60Co is below the limit, contrary to those of 241Am and 137Cs that strongly exceed the limit.
From the radiological protection point of view, adopting the Linear No-Threshold model (LNT), it is required to reduce the activity concentration as much as possible, to limit exposure to ionizing radiation [25]. With this aim, zeolites were used effectively as inorganic ion-exchanger for decontaminating radioactive wastewater. In the following, the features of the main zeolites in adsorption of radionuclides from aqueous solutions are studied and described, perusing through literature [4, 12]. The use of zeolite in the treatment/conditioning of radioactive wastewater from the former nuclear power plant of Latina, in particular liquid supernatant samples from muds of sedimentation of nuclear fuel storage, would fall into the (ii) macro-activities of the decommissioning of the facility (see Sect. 2). The study is hereafter focused on searching for the best zeolites choice suitable for the present purpose of 241Am, 137Cs and 60Co removal from the wastewater samples, exploiting the advances of cations exchange between ions in zeolite structure with radioactive ions in aqueous solutions. The best choice has been made according to the following properties of zeolites: ion exchange capacity, selectivity, thermal and radiation stability, network-like structure and silica-alumina ratio (see Sect. 2.3). The percentage removal (R%) of the specific radionuclide in a sample has been also valued for the choice: R% = (Ci–Cf)/Ci×100, where Ci and Cf are the initial and final radionuclide concentrations, respectively [1]. Americium is a transuranic element of concern in environment, most common oxidation state in aquatic systems and soils. The radioisotope 241Am (half-life = 462 years) is the most prevalent in nuclear wastes as product of high-flux nuclear reactors by two successive neutron captures of 239Pu and the beta decay of the 241Pu. 241Am adsorption from aqueous solutions with zeolites was investigated using: natural zeolites (chabazite, phillipsite, erionite, mordenite and clinoptilolite) and synthetic zeolites (A, X, NaY and L) [26]. Natural zeolites were found poorly efficient < 5%; otherwise the synthetic ones showed, at low pH < 3.5, the percentage removal R% lower than 2%, but R% increases above of 80% at pH > 6.4. Cesium belongs to the alkali metal group, most electropositive, water-soluble. The radioactive isotope 137Cs is a fission product of 235U, and it has a long half-life (about 30 years). Natural zeolites, clinoptilolite (the most abundant in nature), chabazite and natural tuff (montmorillonite, muscovite and heulandite) are good adsorbent for 137Cs in water [27]. Synthetic zeolites, zeolite A (the most used) and zeolite P (synthesized from clinoptilolite and mordenite) show the same good adsorption capability of natural zeolites, having a range of 60–70% percentage of adsorption R% at 7–8.5 pH. Cobalt is a transition metal, monoisotopic in nature. The isotope 59Co forms by (n,γ) reaction 60Co (half-life = 5.2 years). 60Co found in sediments from nuclear facilities. Synthetic zeolite A shows excellent adsorbent properties for the removal of 60Co from aqueous solutions, with an adsorption rate R% of almost 90% under ambient temperature and it increases in alkaline conditions to 98.7% (due to the precipitation of cobalt hydroxide) [5]. The widely used natural zeolites, i.e., clinoptilolite, mordenite, erionite and bentonite, can be also used: the R% increases of 21% when zeolites were treated with NaCl, up to 42% when the temperature was increased to 59°C [4]. Other synthetic zeolites X, NaX and NaY are also used in single- and multi-solute systems at pH 6: the maximum removal rate R% of 68% at 30°C decreases with increasing temperature. The cation exchange processes for the three radionuclides is spontaneous, endothermic and present multilayer adsorption for natural zeolites, monolayer adsorption for synthetic zeolites [12]. Based on these results, the synthetic zeolite A, having a R% ≥ 80%, is the best choice for removing the three radionuclides found in the investigated wastewater. Zeolite A is characterized by the formula |Na+12(H2O)27|8[Al12Si12O48]8 which corresponds to its most common hydrated sodium form. Zeolite A is one of the most common synthetic zeolites, obtained by hydrothermal crystallization of reactive alkali metal aluminosilicate gels at low temperatures (about 100 °C) and pressures (autogenous) under alkaline condition (pH typically higher than 12) [28]. The principal building units of zeolite A are sodalite cages which are connected by four-membered rings forming a three-dimensional network. These cages consist of central cavities of 11.4 A in diameter interconnected by eight-ring openings with a 4.1 A aperture, thus forming a remarkably open zeolite framework with a high void volume fraction of 47% [29]. Zeolite A is easy-to-find commercially (2$/1.5€ per 1 kg) and is commonly used as a desiccant in industrial applications, and especially as processing aid in laundry detergent products, helping manufacturers to produce detergents with excellent physical properties and cleaning performance [28]. Future step of the present work is the setting up of the experimental apparatus to test the use of zeolites in the removal of 241Am, 137Cs and 60Co radionuclides in the wastewater samples from the former Latina nuclear power plant. In-batch experiments by using simulated solution containing not radioactive isotopes and selected zeolites powdered are in progress. The characterization of the exchange process of the zeolites will be completed with dynamic in-column tests, where the liquid passes through. The results obtained will be used for the design and setup of an in-situ pre-pilot plant to carry out tests with contaminated wastewater [10]. This treatment of wastewater produces a radionuclide-bearing zeolite sladge. Radioactive zeolite wastes are harmful for human health and the ecological environment due to their high toxicity and mobility. The inertization of zeolite waste must always aim at their disposal in nature in order to satisfy the demand for a healthy and safe environment as well as comply with national standards [15]. The existing methods for treating zeolite waste include cement and glass–ceramic curing [30]. The first one is relatively simple, low cost, and carried out at room temperature; the disadvantage of this method is the poor compactness, high leaching rate, and larger cement solidification, which increases the treatment cost. Glass–ceramic is used to immobilize radioactive waste due to the advantages of both glass and ceramic, such as reduce nuclide migration, improve the package capacity, and chemical durability of waste. As ultimate goal, the solid cement-like and/or glass-like matrix will be storage at the national repository.
4 Conclusions
As one of the most important adsorbents, zeolite could be used as removal of radionuclides from nuclear wastewater of the former nuclear power plant of Latina, Italy. The wastewater consists of liquid supernatant of mud residuals from the operation of the plant. Samples of the wastewater have been characterized radiologically by performing α- and γ-spectrometry. The results show the presence of 241Am, 137Cs and 60Co radionuclides. Zeolites are microporous material (crystalline) aluminosilicateminerals with structures containing molecular channels and cages that could be accessed by other molecules by ion exchange processes. As reported in the literature, the features of several zeolites, natural and synthetic, have been investigated by examining the percentage removal R% for radionuclides found: synthetic ones, in particular the type A, are expected to be the best solution, for cations such as 241Am, 60Co and 137Cs. This work falls within the decommissioning of a nuclear facility, by proposing an alternative and new solution for correctly managing the problem of radioactive waste treatment. Evolutions of the project are working in progress, by setting up equipments suitable for the materials to be treated and the processes to be implemented to test the use of zeolites in the radionuclides removal. Once the method of radionuclides removal from wastewater by zeolites has been standardized, it can be extended to all the similar industrial realities.
Data Availability Statement
This manuscript has associated data in a data repository. [Authors’ comment: Data will be made available on reasonable request].
References
S. Kwon, S. Kim, E. Han, H. Lee, H.S. Cho, M. Choi, Relationship between zeolite structure and capture capability for radioactive cesium and strontium. J. Hazard. Mater. 408, 124419 (2021)
D. Delacroix, J.P. Guerre, P. Leblanc, C. Hickman, Radionuclide and radiation protection data handbook 2002. Radiat. Prot. Dosim. 98, 1–168 (2002)
G. Patzay, P. Tilky, J. Schunk, T. Pinter, F. Feil, K. Hamaguchi, L. Weiser, Radioactive wastewater treatment using a mixture of TANNIX sorbent and VARION mixed bed ion exchange resin. Int. J. Nucl. Energy Sci. Technol. 2, 328 (2006)
M. Jimenez-Reyes, P.T. Almazan-Sanchez, M. Solache-Ríos, Radioactive waste treatments by using zeolites. A short review. J. Environ. Radioact. 233, 106610 (2021)
X.H. Fang, F. Fang, C.H. Lu, L. Zheng, Removal of Cs+, Sr2+, and Co2+ ions from the mixture of organics and suspended solids aqueous solutions by zeolites. Nucl. Eng. Technol. 49, 556–561 (2017)
A.K. Singh, A. Kumar, R. Chandra, Environmental pollutants of paper industry wastewater and their toxic effects on human health and ecosystem. Bioresour. Technol. Rep. 20, 101250 (2022)
Euratom 2013, European Directive 59/2013/EURATOM-Basic safety standards for protection against the dangers arising from exposure to ionising radiation. OJ of the EU. L13 2014, 57, 1–73 (2013)
R.O.A. Rahman, H.A. Ibrahium, Y. Hung, Liquid radioactive wastes treatment—a review. Water 3, 551–565 (2011)
M.H. Mallah, H. Soorchi, T.F. Jooybari, Development of empirical equation for analcime in the treatment of nuclear waste. Ann. Nucl. Energy 47, 140–145 (2012)
D. Caputo, F. Pepe, Experiments and data processing of ion exchange equilibria involving Italian natural zeolites—a review. Microporous and Mesoporous Mater. 105(3), 222–231 (2007)
B. Liguori, D. Caputo, F. Iucolano, P. Aprea, B. de Gennaro, Entrapping of Cs and Sr in heat-treated zeolite matrices. J. Nucl. Mater. 435, 196–201 (2013)
A. Khaleque, M.M. Alam, M. Hoque, S. Mondal, J.B. Haider, B. Xu, M.A. Johir, A.K. Karmakar, J.L. Zhou, M.B. Ahmed, M.A. Moni, Zeolite synthesis from low-cost materials and environmental applications—a review. Environ. Adv. 2, 100019 (2020)
G. Canzone, R. Lo Frano, M. Sumini, F. Troiania, Dismantling of the graphite pile of Latina NPP: characterization and handling/removal equipment for single brick or multi-bricks. Prog. Nucl. Energy 93, 146–154 (2016)
Mise, Istanza Autorizzativa LT G 00006, Piano Globale di Disattivazione Accelerata-FASE 1, volume I e III, Rev. 02 (2010)
D.lgs 101/2020, Implementation of Directive 2013/59/EURATOM. Gazze. Uff. 201(29/L), Roma, Italy, 348 (2020)
D.lgs 31/2010, Disciplina dei sistemi di stoccaggio del combustibile irraggiato e rifiuti radioattivi. Gazze. Uff. 55(10G0048), Roma, Italy, 48 (2010)
C. Colella, Natural zeolites. Stud. Surf. Sci. Catal. 157, 13–40 (2005)
C. Baerlocher, L.B. McCusker, D.H. Olson, Atlas of Zeolite Framework Types, 6th edn. (Elsevier, Amsterdam, 2007)
P. Aprea, B. De Gennaro, N. Gargiulo, A. Peluso, B. Liguori, F. Iucolano, D. Caputo, Sr-, Zn- and Cd-exchanged zeolitic materials as water vapor adsorbents for thermal energy storage applications. Appl. Therm. Eng. 106, 1217–1224 (2016)
Y. Türkmen, M. Özdemir, F. Kurudirek, Ö. Demir, R. Simsek, Demirboğa, Calculation of radiation attenuation coefficients in Portland cements mixed with silica fume, blast furnace slag and natural zeolite. Ann. Nucl. Energy 35(10), 1937–1943 (2008)
Y. Li, L. Li, J. Yu, Applications of zeolites in sustainable chemistry. Inside Chem. 3(6), 928–949 (2017)
P. Misaelides, Application of natural zeolites in environmental remediation: a short review. Microporous Mesoporous Mater. 144(1–3), 15–18 (2011)
F. Ambrosino, L. Stellato, C. Sabbarese, A case study on possible radiological contamination in the Lo Uttaro landfill site (Caserta, Italy). J. Phys: Conf. Series 1548(1), 012001 (2020)
L. Rinaldi, F. Ambrosino, V. Roca, A. D’Onofrio, C. Sabbarese, Study of 222–220Rn measurement systems based on electrostatic collection by using geant4+COMSOL Simulation. Appl. Sci. 12, 507 (2022)
ICRP 2007, The 2007 recommendations of the international commission on radiological protection. ICRP Publication 103. Ann. ICRP 37 (2–4) (2007)
H. Mimura, Y. Ishihara, K. Akiba, Adsorption behavior of americium on zeolites. J. Nucl. Sci. Technol. 28(2), 144–151 (1991)
N.V. Elizondo, E. Ballesteros, B.I. Kharisov, Cleaning of liquid radioactive wastes using natural zeolites. Appl. Radiat. Isot. 52(1), 27–30 (2000)
J. Yu, Synthesis of zeolites. In: J. Cejka, H. van Bekkum, A. Corma, F. Schueth (eds) Introduction to zeolite molecular sieves, studies in surface science and catalysis, Elsevier, Amsterdam, 168. pp 39–104 (2007)
A. Julbe, M. Drobek, A. Zeolite, in Encyclopedia of Membranes. ed. by E. Drioli, L. Giorno (Springer, Berlin, Heidelberg, 2016)
B. Yuan, F. Luo, Y. Miao, M. Shi, Y. Zhao, W. Huang, Z. Xu, Lu X Immobilization of simulated An4+ radioactively contaminated zeolite: solidify mechanism and theory investigation. J. Solid State Chem. 311, 123095 (2022)
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ambrosino, F., Esposito, A.M., Mancini, F. et al. Zeolites identification for wastewater radionuclides removal in the decommissioning of a former Italian nuclear power plant. Eur. Phys. J. Plus 138, 909 (2023). https://doi.org/10.1140/epjp/s13360-023-04491-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-023-04491-3