Abstract—
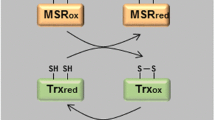
The conversion of methionine to methionine sulfoxide (MetO) is one of the most common oxidative modifications in proteins due to the special susceptibility of methionine to oxidative conditions. Methionine oxidation can affect the protein structure and function, while the level of MetO increases with the development of oxidative stress. Most cells contain methionine sulfoxide reductases (MSRs), which catalyze a thioredoxin-dependent reduction of methionine sulfoxide to the original methionine. It was demonstrated that mutations leading to a decrease in MSR activity are associated with a decrease in the resistance of some cells to oxidative stress, while mutations leading to an overproduction of MSR activity result in an increase in resistance to oxidative stress. The redox reactions of methionines in the functional regulation of some intracellular proteins, actin, and calmodulin, are analyzed in the work, and the presence of antioxidant methionines in intracellular proteins, such as glutamine synthetase, 15-lipoxygenase, recombinant proteins, interferon α-2b, tissue plasminogen activator, and human stem cell factor, is discussed. The absence of MSR in the blood plasma makes the oxidation of methionines in the proteins irreversible; therefore, the ability of methionines to serve as interceptors of oxidant molecules without impairment of the function of plasma proteins is quite controversial. Antioxidant methionines were found in a number of proteins, such as macroglobulin, antithrombin III, and blood coagulation factor XIII. However, no antioxidant methionines were detected for most blood plasma proteins. There is a correlation between the oxidation of methionines and the development of pathological conditions in the organism.





Similar content being viewed by others
REFERENCES
Aledo, J.C., Methionine in proteins: the Cinderella of the proteinogenic amino acids, Protein Sci., 2019, vol. 28, pp. 1785–1796.
Aledo, J.C., Li, Y., de Magalhães, J.P., et al., Mitochondrially encoded methionine is inversely related to longevity in mammals, Aging Cell, 2011, vol. 10, pp. 198–207.
Aledo, J.C., Valverde, H., and De Magalhães, J.P., Mutational bias plays an important role in shaping longevity-related amino acid content in mammalian mtDNA-encoded proteins, J. Mol. Evol., 2012, vol. 74, pp. 332–341.
Aledo, J.C., Cantón, F.R., and Veredas, F.J., Sulphur atoms from methionines interacting with aromatic residues are less prone to oxidation, Sci. Rep., 2015, vol. 5, p. 16955.
Bagoly, Z. and Muszbek, L., Factor XIII: what does it look like? J. Thromb. Haemostasis, 2019, vol. 17, pp. 714–716.
Barbato, G., Ikura, M., Kay, L.E., et al., Backbone dynamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy: the central helix is flexible, Biochemistry, 1992, vol. 31, vol. 5269–5278.
Bender, A., Hajieva, P., and Moosmann, B., Adaptive antioxidant methionine accumulation in respiratory chain complexes explains the use of a deviant genetic code in mitochondria, Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, pp. 16496–16501.
Bruschi, M., Candiano, G., Santucci, L., and Ghiggeri, G.M., Oxidized albumin. The long way of a protein of uncertain function, Biochim. Biophys. Acta, Gen. Subj., 2013, vol. 1830, pp. 5473–5479.
Burney, P.R., White, N., and Pfaendtner, J., Structural effects of methionine oxidation on isolated subdomains of human fibrin D and αC regions, PLoS One, 2014, vol. 9, pp. 1–10.
Carocho, M. and Ferreira, I.C., A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives, Food Chem. Toxicol., 2013, vol. 51, pp. 15–25.
Carrell, N.A., Erickson, H.P., and McDonagh, J., Electron microscopy and hydrodynamic properties of factor XIII subunits, J. Biol. Chem., 1989, vol. 264, pp. 551–556.
Carrell, R.W., Stein, P.E., Fermi, G., and Wardell, M.R., Biological implications of a 3 Å structure of dimeric antithrombin, Structure, 1994, vol. 2, pp. 257–270.
Cater, J.H., Wilson, M.R., and Wyatt, A.R., Alpha-2-macroglobulin, a hypochlorite-regulated chaperone and immune system modulator, Oxid. Med. Cell. Longevity, 2019, vol. 2019, art. ID 5410657.
Chao, C.C., Ma, Y.S., and Stadtman, E.R., Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems, Proc. Natl. Acad. Sci. U.S.A., 1997, vol. 94, pp. 2969–2974.
Chen, B., Mayer, M.U., and Squier, T.C., Structural uncoupling between opposing domains of oxidized calmodulin underlies the enhanced binding affinity and inhibition of the plasma membrane Ca-ATPase, Biochemistry, 2005, vol. 44, pp. 4737–4747.
Chin, D. and Means, A.R., Calmodulin: a prototypical calcium sensor, Trends Cell Biol., 2000, vol. 10, pp. 322–328.
Chyan, C.-L., Irene, D., and Lin, S.-M., The recognition of calmodulin to the target sequence of calcineurin—a novel binding mode, Molecules, 2017, vol. 22, p. 1584.
Cobley, J.N. Mechanisms of mitochondrial ROS production in assisted reproduction: the known, the unknown, and the intriguing, Antioxidants (Basel), 2020, vol. 9, p. 933.
Dahl, J.U., Gray, M.J., and Jakob, U., Protein quality control under oxidative stress conditions, J. Mol. Biol., 2015, vol. 427, pp. 1549–1563.
Davies, M.J., Protein oxidation and peroxidation, Biochem. J., 2016, vol. 473, pp. 805–825.
De Cristofaro, R. and Landolfi, R., Oxidation of human alpha-thrombin by the myeloperoxidase-H2O2-chloride system: structural and functional effects, Thromb. Haemostasis, 2000, vol. 83, pp. 253–261.
De Vries, J.J., Snoek, C.J.M., Rijken, D.C., and de Maat, M.P.M., Effects of post-translational modifications of fibrinogen on clot formation, clot structure, and fibrinolysis, Arterioscler., Thromb. Vasc. Biol., 2020, vol. 40, pp. 554–569.
Douglas, T., Daniel, D.S., Parida, B.K., et al., Methionine sulfoxide reductase A (MsrA) deficiency affects the survival of Mycobacterium smegmatis within macrophages, J. Bacteriol., 2004, vol. 186, pp. 3590–3598.
Drazic, A. and Winter, J., The physiological role of reversible methionine oxidation, Biochim. Biophys. Acta, Proteins Proteomics, 2014, vol. 1844, pp. 1367–1382.
Elmallah, M.I., Borgmeyer, U., Betzel, C., and Redecke, L., Impact of methionine oxidation as an initial event on the pathway of human prion protein conversion, Prion, 2013, vol. 7, pp. 404–411.
Gan, Q.F., Witkop, G.L., Sloane, D.L., et al., Identification of a specific methionine in mammalian 15-lipoxygenase which is oxygenated by the enzyme product 13-HPODE: dissociation of sulfoxide formation from self-inactivation, Biochemistry, 1995, vol. 34, pp. 7069–7079.
Garner, B., Waldeck, A.R., Witting, P.K., et al., Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII, J. Biol. Chem., 1998, vol. 273, pp. 6088–6095.
Gellman, S.H., On the role of methionine residues in the sequence independent recognition of nonpolar protein surfaces, Biochemistry, 1991, vol. 30, pp. 6633–6636.
Gifford, J.L., Walsh, M.P., and Vogel, H.J., Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs, Biochem. J., 2007, vol. 405, pp. 199–221.
Gray, E. and Barrowcliffe, T.W., Inhibition of antithrombin III by lipid peroxides, Thromb. Res., 1985, vol. 37, pp. 241–250.
Griffiths, H.R., Dias, I.H., Willetts, R.S., and Devitt, A., Redox regulation of protein damage, Redox Biol., 2014, vol. 2, pp. 430–435.
Grintsevich, E.E., Ge, P., Sawaya, M.R., et al., Catastrophic disassembly of actin filaments via Mical-mediated oxidation, Nat. Commun., 2017, vol. 8, p. 2183.
Grintsevich, E.E., Yesilyurt, H.G., Rich, S.K., et al., F-actin dismantling through a redox-driven synergy between Mical and cofilin, Nat. Cell. Biol., 2016, vol. 18, vol. 876–885.
Grivennikova, V.G. and Vinogradov, A.D., Generation of reactive oxygen species by mitochondria, Usp. Biol. Khim., 2013, vol. 53, pp. 245–296.
Grune, T., Reinheckel, T., and Davies, K.J., Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome, J. Biol. Chem., 1996, vol. 271, pp. 15504–15509.
Grune, T., Catalgol, B., and Jung, T., Protein Oxidation and Aging, Chichester: Wiley, 2013.
Gu, S.X., Stevens, J.W., and Lentz, S.R., Regulation of thrombosis and vascular function by protein methionine oxidation, Blood, 2015, vol. 125, pp. 3851–3859.
Gutmann, C., Siow, R., Gwozdz, A.M., et al., Reactive oxygen species in venous thrombosis, Int. J. Mol. Sci., 2020, vol. 21, p. 1918.
Hawkins, C.L., Pattison, D.I., and Davies, M.J., Hypochlorite-induced oxidation of amino acids, peptides and proteins, Amino Acids, 2003, vol. 25, pp. 259–274.
Hsu, Y.R., Narhi, L.O., and Spahr, C., In vitro methionine oxidation of Escherichia coli-derived human stem cell factor: effects on the molecular structure, biological activity, and dimerization, Protein Sci., 1996, vol. 5, pp. 1165–1173.
Hung, R.-J., Park, C.W., and Terman, J.R., Direct redox regulation of F-actin assembly and disassembly by Mical, Science, 2011, vol. 334, pp. 1710–1714.
Hung, R.-J., Spaeth, C.S., Yesilyurt, H.G., and Terman, J.R., SelR reverses Mical-mediated oxidation of actin to regulate F-actin dynamics, Nat. Cell. Biol., 2013, vol. 15, pp. 1445–1454.
Karbyshev, M.S. and Abdullaev, Sh.P., Biokhimiya oksidativnogo stressa. Okislitel’nyi stress. Prooksidanty i antioksidanty (Biochemistry of Oxidative Stress. Oxidative Stress. Prooxidants and Antioxidants), Moscow, 2018.
Kattula, S., Byrnes, J.R., and Wolberg, A.S., Fibrinogen and fibrin in hemostasis and thrombosis, Arterioscler., Thromb., Vasc. Biol., 2017, vol. 37, p. e13–e21.
Keck, R.G., The use of t-butyl hydroperoxide as a probe for methionine oxidation in proteins, Anal. Biochem., 1996, vol. 236, pp. 56–62.
Khan, S.A. and Khan, F.H., Hydroxyl radical mediates oxidative modification of caprine alpha-2-macroglobulin, Protein Pept. Lett., 2009, vol. 16, pp. 32–35.
Lankin, V.Z., Tikhaze, A.K., and Belenkov, Yu.N., Svobodnoradikal’nye protsessy v norme i pri patologicheskikh sostoyaniyakh (posobie dlya vrachei) (Free Radical Processes in Normal and Pathological Conditions: Manual for Physicians), Moscow: Nats. Med. Issled. Tsentr Kardiol., Minist. Zdravookhr. RF, 2001.
Le-Donne, I., Rossi, R., Milzani, A., et al., The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself, Free Radical Biol. Med., 2001, vol. 31, pp. 1624–1632.
Lim, J.C., You, Z., Kim, G., and Levine, R.L., Methionine sulfoxide reductase A is a stereospecific methionine oxidase, Proc. Natl. Acad. Sci. U.S.A., 2011, vol. 108, pp. 10472–10477.
Lim, J.C., Kim, G., and Levine, R.L., Stereospecific oxidation of calmodulin by methionine sulfoxide reductase A, Free Radical Biol. Med., 2013, vol. 61, pp. 257–264.
Lim, J., Kim, G., and Levine, R., Methionine in proteins: it’s not just for protein initiation anymore, Neurochem. Res., 2019, vol. 44, pp. 1–11.
Lishko, V.K., Podolnikova, N.P., Yakubenko, V.P., et al., Multiple binding sites in fibrinogen for integrin αMβ2 (Mac-1), J. Biol. Chem., 2004, vol. 279, pp. 44897–44906.
Liu, F., Chu, X., Lu, H.P., and Wang, J., Molecular mechanism of multispecific recognition of calmodulin through conformational changes, Proc. Natl. Acad. Sci. U.S.A., 2017, vol. 114, pp. E3927–E3934.
Lu, A.L., Li, X., Gu, Y., et al., Repair of oxidative DNA damage: mechanisms and functions, Cell. Biochem. Biophys., 2001, vol. 35, pp. 141–170.
Manta, B. and Gladyshev, V.N., Regulated methionine oxidation by monooxygenases, Free Radical Biol. Med., 2017, vol. 109, pp. 141–155.
Marimoutou, M., Springer, D.A., Liu, C., et al., Oxidation of methionine 77 in calmodulin alters mouse growth and behavior, Antioxidants (Basel), 2018, vol. 7, no. 10, p. 140.
Marrero, A., Duquerroy, S., Trapani, S., et al., The crystal structure of human α2-macroglobulin reveals a unique molecular cage, Angew. Chem., 2012, vol. 51, pp. 3340–3344.
Martinez, M., Weisel, J.W., and Ischiropoulos, H., Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots, Free Radical Biol. Med., 2013, vol. 65, pp. 411–418.
McCarthy, M.R., Thompson, A.R., Nitu, F., et al., Impact of methionine oxidation on calmodulin structural dynamics, Biochem. Biophys. Res. Commun., 2015, vol. 456, pp. 567–572.
Men’shchikova, E.B., Lankin, V.Z., Zenkov, N.K., et al., Okislitel’nyi stress. Prooksidanty i antioksidanty (Oxidative Stress. Prooxidants and Antioxidants), Moscow: Slovo, 2006.
Milzani, A., Rossi, R., Di Simplicio, P., et al., The oxidation produced by hydrogen peroxide on Ca-ATP-G-actin, Protein Sci., 2000, vol. 9, pp. 1774–1782.
Misztal, T., Golaszewska, A., Tomasiak-Lozowska, M.M., et al., The myeloperoxidase product, hypochlorous acid, reduces thrombus formation under flow and attenuates clot retraction and fibrinolysis in human blood, Free Radical Biol. Med., 2019, vol. 141, pp. 426–437.
Moskovitz, J., Berlett, B.S., Poston, J.M., and Stadtman, E.R., The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo, Proc. Natl. Acad. Sci. U.S.A., 1997, vol. 94, pp. 9585–9589.
Moskovitz, J., Flescher, E., Berlett, B.S., et al., Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress, Proc. Natl. Acad. Sci. U.S.A., 1998, vol. 95, pp. 14071–14075.
Moskovitz, J., Levine, R.L., and Stadtman, E.R., Oxidation of methionine in proteins roles in antioxidant defense and cellular regulation, IUBMB Life, 2000, vol. 50, pp. 301–317.
Moskovitz, J., Bar-Noy, S., Williams, W.M., et al., Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals, Proc. Natl. Acad. Sci. U.S.A., 2001, vol. 98, pp. 12920–12925.
Nimalaratne, C. and Wu, J., Hen egg as an antioxidant food commodity: a review, Nutrients, 2015, vol. 7, pp. 8274–8293.
Nishinaka, Y., Masutani, H., Nakamura, H., and Yodoi, J., Regulatory roles of thioredoxin in oxidative stress-induced cellular responses, Redox Rep., 2001, vol. 6, pp. 289–295.
Oda, T., Iwasa, M., Aihara, T., et al., The nature of the globular- to fibrous-actin transition, Nature, 2009, vol. 457, pp. 441–445.
Olson, S.T. and Björk, I., Regulation of thrombin activity by antithrombin and heparin, Sem. Thromb. Haemostasis, 1994, vol. 20, pp. 373–409.
Olson, S.T., Swanson, R., Raub-Segall, E., et al., Accelerating ability of synthetic oligosaccharides on antithrombin inhibition of proteinases of the clotting and fibrinolytic systems. Comparison with heparin and low-molecular-weight heparin, Thromb. Haemostasis, 2004, vol. 92, pp. 929–939.
Panasenko, O.M., Gorudko, I.V., and Sokolov, A.V., Hypochlorous acid as a precursor of free radicals in living systems, Usp. Biol. Khim., 2013, vol. 53, pp. 195–244.
Pederson, E.N. and Interlandi, G., Oxidation-induced destabilization of the fibrinogen αC-domain dimer investigated by molecular dynamics simulations, Proteins, 2019, vol. 87, pp. 826–836.
Poljšak, B. and Fink, R., The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution, Oxid. Med. Cell. Longevity, 2014, vol. 2014, pp. 1–22.
Protopopova, A.D., Ramirez, A., Klinov, D.V., et al., Factor XIII topology: organization of B subunits and changes with activation studied with single-molecule atomic force microscopy, J. Thromb. Haemostasis, 2019, vol. 17, pp. 737–748.
Qin, Z. and Squier, T.C., Calcium-dependent stabilization of the central sequence between Met(76) and Ser(81) in vertebrate calmodulin, Biophys. J., 2001, vol. 81, pp. 2908–2918.
Rau, J.C., Beaulieu, L.M., Huntington, J.A., and Church, F.C., Serpins in thrombosis, hemostasis and fibrinolysis, J. Thromb. Haemostasis, 2007, vol. 5, suppl. 1, pp. 102–115.
Ray, P.D., Huang, B.-W., and Tsuji, Y., Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling, Cell. Signaling, 2012, vol. 24, pp. 981–990.
Reddy, V.Y., Desorchers, P.E., Pizzo, S.V., et al., Oxidative dissociation of human α2-macroglobulin tetramers into dysfunctional dimers, J. Biol. Chem., 1994, vol. 269, pp. 4683–4691.
Rehman, A.A., Ahsan, H., and Khan, F.H., α2-Macroglobulin: a physiological guardian, J. Cell. Physiol., 2013, vol. 8, pp. 1665–1675.
Rijken, D.C. and Uitte de Willige, S., Inhibition of fibrinolysis by coagulation factor XIII, Biomed. Res. Int., 2017, vol. 2017, art. ID 1209676.
Rizzo, A.M., Berselli, P., Zava, S., et al., Endogenous antioxidants and radical scavengers, Adv. Exp. Med. Biol., 2010, vol. 698, pp. 52–67.
Rivett, A.J., Regulation of intracellular protein turnover: covalent modification as a mechanism of marking proteins for degradation, Curr. Top. Cell. Reg., 1986, vol. 28, pp. 291–337.
Rosenfeld, M.A., Bychkova, A.V., Shchegolikhin, A.N., et al., Ozone-induced oxidative modification of plasma fibrin-stabilizing factor, Biochim. Biophys. Acta, Proteins Proteomics, 2013, vol. 1834, pp. 2470–2479.
Rosenfeld, M.A, Vasilyeva, A.D., Yurina, L.V., et al., Oxidation of proteins: is it a programmed process? Free Radical Res., 2018, vol. 52, pp. 14–38.
Ruan, H., Tang, X.D., Chen, M.L., et al., High-quality life extension by the enzyme peptide methionine sulfoxide reductase, Proc. Natl. Acad. Sci. U.S.A., 2002, vol. 99, pp. 2748–2753.
Shacter, E., Williams, J.A., and Lim, M., Differential susceptibility of plasma proteins to oxidative modification: examination by Western blot immunoassay, Free Radical Biol. Med., 1994, vol. 17, pp. 429–436.
Sigalov, A.B. and Stern, L.J., Enzymatic repair of oxidative damage to human apolipoprotein A-I, FEBS Lett., 1998, vol. 433, pp. 196–200.
Simiczyjew, A., Mazur, A.J., Dratkiewicz, E., and Nowak, D., Involvement of β- and γ-actin isoforms in actin cytoskeleton organization and migration abilities of bleb-forming human colon cancer cells, PLoS One, 2017, vol. 12, p. e0173709.
Souri, M., Kaetsu, H., and Ichinose, A., Sushi domains in the subunit-B of factor XIII responsible for oligomer assembly, Biomolecules, 2008, vol. 47, pp. 8656–8664.
Souri, M., Osaki, T., and Ichinose, A., The non-catalytic B subunit of coagulation actor XIII accelerates fibrin cross-linking, J. Biol. Chem., 2015, vol. 290, pp. 12027–12039.
Sovová, Ž., Štikarová, J., Kaufmanová, J., et al., Impact of posttranslational modifications on atomistic structure of fibrinogen, PLoS One, 2020, vol. 15, p. e0227543.
Spahr, C.S., Nahri, L., Speakman, J., et al., The effects of in vitro methionine oxidation on the bioactivity and structure of human keratinocyte growth factor, Protein Sci., 1996, vol. 5, pp. 299–308.
Stadtman, E.R. and Levine, R.L., Free radical-mediated oxidation of free amino acids and amino acid residues in proteins, Amino Acids, 2003, vol. 25, pp. 207–218.
Stief, T.W., Aab, A., and Heimburger, N., Oxidative inactivation of purified human alpha-2-antiplasmin, antithrombin III, and C1-inhibitor, Thromb. Res., 1988, vol. 49, pp. 581–589.
Stief, T.W., Kurz, J., Doss, M.O., and Fareed, J., Singlet oxygen inactivates fibrinogen, factor V, factor VIII, factor X, and platelet aggregation of human blood, Thromb. Res., 2000, vol. 97, pp. 473–480.
Stournaras, C., Drewes, G., Blackholm, H., et al., Glutathionyl(cysteine-374) actin forms filaments of low mechanical stability, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1990, vol. 1037, pp. 86–91.
Tarrago, L., Kaya, A., Weerapana, E., et al., Methionine sulfoxide reductases preferentially reduce unfolded oxidized proteins and protect cells from oxidative protein unfolding, J. Biol. Chem., 2012, vol. 287, pp. 24448–24459.
Tebar, F., Chavero, A., Agell, N., et al., Pleiotropic roles of calmodulin in the regulation of KRas and Rac1 GTPases: functional diversity in health and disease, Int. J. Mol. Sci., 2020, vol. 21, p. 3680.
Tse, G., Yan, B.P., Chan, Y.W., et al., Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis, Front. Physiol., 2016, vol. 7, p. 313.
Tsurupa, G., Pechik, I., Litvinov, R.I., et al., On the mechanism of αC polymer formation in fibrin, Biochemistry, 2012, vol. 51, pp. 2526–2538.
van Patten, S.M., Hanson, E., Bernasconi, R., et al., Oxidation of methionine residues in antithrombin. Effects on biological activity and heparin binding, J. Biol. Chem., 1999, vol. 274, pp. 10268–10276.
Vasilyeva, A., Indeykina, M., Bychkova, A., et al., Oxidation-induced modifications of the catalytic subunits of plasma fibrin-stabilizing factor at the different stages of its activation identified by mass spectrometry, Biochim. Biophys. Acta, Proteins Proteomics, 2018, vol. 1866, pp. 875–884.
Vasilyeva, A., Yurina, L., Shchegolikhin, A., et al., The structure of blood coagulation factor XIII is adapted to oxidation, Biomolecules, 2020, vol. 10, p. 914.
Veredas, F.J., Aledo, J.C., and Cantón, F.R., Methionine residues around phosphorylation sites are preferentially oxidized in vivo under stress conditions, Sci. Rep., 2017, vol. 7, p. 40403.
Vetter, S.W. and Leclerc, E., Novel aspects of calmodulin target recognition and activation, Eur. J. Biochem., 2003, vol. 270, pp. 404–414.
Vogt, W., Oxidation of methionyl residues in proteins: tools, targets, and reversal, Free Radical Biol. Med., 1995, vol. 18, pp. 93–105.
Walgenbach, D.G., Gregory, A.J., and Klein, J.C., Unique methionine-aromatic interactions govern the calmodulin redox sensor, Biochem. Biophys. Res. Commun., 2018, vol. 20, pp. 236–241.
Walker E.J., Bettinger J.Q., Welle K.A., et al., Global analysis of methionine oxidation provides a census of folding stabilities for the human proteome, Proc. Natl. Acad. Sci. U.S.A., 2019, vol. 116, pp. 6081–6090.
Wang, J., Boja, E.S., Tan, W., et al., Reversible glutathionylation regulates actin polymerization in A431 cells, J. Biol. Chem., 2001, vol. 276, pp. 47763–47766.
Wang, P., Wu, Y., Li, X., et al., Thioredoxin and thioredoxin reductase control tissue factor activity by thiol redox-dependent mechanism, J. Biol. Chem., 2013, vol. 288, pp. 3346–3358.
Wang, Q. and Zennadi, R., Oxidative stress and thrombosis during aging: the roles of oxidative stress in RBCS in venous thrombosis, Int. J. Mol. Sci., 2020, vol. 21, p. 4259.
Wang, Z., Feng, B., Xiao, G., and Zhou, Z., Roles of methionine oxidation in E200K prion protein misfolding: implications for the mechanism of pathogenesis in E200K linked familial Creutzfeldt–Jakob disease, Biochim. Biophys. Acta, Proteins Proteomics, 2016, vol. 1864, pp. 346–358.
Weigandt, K.M., White, N., Chung, D., et al., Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen, Biophys. J., 2012, vol. 103, pp. 2399–2407.
Weisel, J.W. and Litvinov, R.I., Fibrin formation, structure and properties, Subcell. Biochem., 2017, vol. 82, pp. 405−456.
Weissbach, H., Resnick, I., and Brot, N., Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage, Biochim. Biophys. Acta, Proteins Proteomics, 2005, vol. 1703, pp. 203–212.
White, N.J., Wang, Y., Fu, X., et al., Post-translational oxidative modification of fibrinogen is associated with coagulopathy after traumatic injury, Free Radical Biol. Med., 2016, vol. 96, pp. 181–189.
Wilson, C., Terman, J.R., González-Billault, C., and Ahmed, G., Actin filaments—a target for redox regulation, Cytoskeleton, 2016, vol. 73, pp. 577–595.
Wu, S.M., Patel, D.D., and Pizzo, S.V., Oxidized α2-macroglobulin (α2M) differentially regulates receptor binding by cytokines/growth factors: implications for tissue injury and repair mechanisms in inflammation, J. Immunol., 1998, vol. 161, pp. 4356–4365.
Wyatt, A.R., Kumita, J.R., Farrawell, N.E., et al., Alpha-2-macroglobulin is acutely sensitive to freezing and lyophilization: implications for structural and functional studies, PLoS One, 2015, vol. 10, p. e0130036.
Wyatt, A.R., Kumita, J.R., Mifsud, R.W., et al., Hypochlorite-induced structural modifications enhance the chaperone activity of human α2-macroglobulin, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, pp. 2081–2090.
Xu, K., Uversky, V.N., and Xue, B., Local flexibility facilitates oxidization of buried methionine residues, Protein Pept. Lett., 2012, vol. 19, pp. 688–697.
Xu, Q., Huff, L.P., Fujii, M., and Griendling, K.K., Redox regulation of the actin cytoskeleton and its role in the vascular system, Free Radical Biol. Med., 2017, vol. 109, pp. 84–107.
Yamniuk, A.P. and Vogel, H.J., Calmodulin’s flexibility allows for promiscuity in its interactions with target proteins and peptides, Mol. Biotechnol., 2004, vol. 27, pp. 33–57.
Yermolaieva, O., Xu, R., Schinstock, C., et al., Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation, Proc. Natl. Acad. Sci. U.S.A., 2004, vol. 101, pp. 1159–1164.
Yurina, L.V., Vasilyeva, A.D., Bugrova, A.E., et al., Hypochlorite-induced oxidative modification of fibrinogen, Dokl. Biochem. Biophys., 2019a, vol. 484, no. 1, pp. 37–41.
Yurina, L., Vasilyeva, A., Indeykina, M., et al., Ozone-induced damage of fibrinogen molecules: identification of oxidation sites by high-resolution mass spectrometry, Free Radical Res., 2019b, vol. 53, pp. 430–455.
Yurina, L.V., Vasilyeva, A.D., Kononenko, V.L., et al., The structural-functional damage of fibrinogen oxidized by hydrogen peroxide, Dokl. Biochem. Biophys., 2020, vol. 492, no. 1, pp. 130–134.
ACKNOWLEDGMENTS
The authors are grateful to anonymous reviewer for valuable comments that contributed to improvement of the quality of publication.
Funding
This work was supported in part by the Russian Foundation for Basic Research, project no. 18-04-01313.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Rosenfeld, M.A., Yurina, L.V. & Vasilyeva, A.D. Functional Role of Methionine Oxidation in Proteins: Arguments for and against. Biol Bull Rev 11 (Suppl 1), 1–18 (2021). https://doi.org/10.1134/S2079086421070070
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086421070070