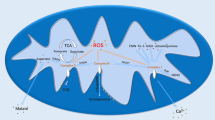
Abstract
The development of oxidative stress inevitably causes damage to cell proteins in need of timely refolding. The synthesis of the proteins of the heat-shock group is the most ancient and effective mechanism of cell protection. The life-long cellular adaptation to a huge number of cytotoxic factors, both of xenobiotic and natural origin, have provided heat-shock proteins with polyfunctionality: in the processes of programmed death, they act as either anti- or pro-apoptogenic factors or as regulators of the activity of these factors. The important role of heat-shock proteins in adaptation, inflammation, and immune response had also been shown. Various experimental and clinical studies confirmed the important role of heat-shock proteins in the development of pathophysiological phenomena of oxidative stress, aging, tumor formation, and immune reactions. The data presented in the review includes domestic and foreign studies on the physiological significance of heat-shock proteins, their role in the mechanisms of cell, and body stability in general and are relevant to modern medicine and biology.
Similar content being viewed by others
REFERENCES
Andreeva, L.I., Theoretical and applied value of 70 kDa heat shock proteins: the possible practical application and pharmacological correction, Obzory Klin. Farmakol. Lek. Ter., 2002, vol. 1, no. 2, pp. 2–14.
Grigorian, I.Yu., Linkova, N.S., Polyakova, V.O., et al., Signaling molecules of the endometrium: gerontological and general pathological aspects, Adv. Gerontol., 2016, vol. 6, no. 1, pp. 36–43.
Kabalyk, M.A., Age-related aspects of the involvement of heat shock proteins in the pathogenesis of osteoarthritis, Adv. Gerontol., 2017, vol. 7, no. 4, pp. 276–280.
Kabalyk, M.A., Clinical and pathogenetic role of 70- and 27-kDa heat shock proteins in osteoarthritis, Nauchno-Prakt.Revmatol., 2017, vol. 55, no. 2, pp. 187–191.
Kolesov, S.A., Zhukova, E.A., Korkotashvili, L.V., et al., Nitric oxide metabolites, heat shock proteins 70, proinflammatory cytokines in children with inflammatory bowel diseases, Mezhdunar. Zh. Prikl. Fundam. Issled., 2014, no. 4, pp. 75–78.
Kolesnikova, L.I., Kurashova, N.A., Grebenkina, L.A., et al., Features of oxidative stress in men of different ethnic groups with obesity and infertility, Zdorov’e. Med. Ekol. Nauka, 2011, vol. 44, no. 1, pp. 38–41.
Kolesnikova, L.I., Osipova, E.V., and Grebenkina, L.A., Okislitel’nyi stress pri reproduktivnykh narusheniyakh endokrinnogo geneza u zhenshchin (Oxidative Stress in Reproductive Disorders of Endocrine Genesis in Women), Novosibirsk, 2011.
Kuznik, B.I., Linkova, N.S., and Khavinson, V.K., Heat shock proteins: changes related to aging, development of thrombotic complications, and peptide regulation of the genome, Adv. Gerontol., 2012, vol. 2, no. 3, pp. 175–186.
Kuznik, B.I., Khavinson, V.Kh., Lin’kova, N.S., et al., Fibroblast growth factors FGF19, FGF21, FGF23 as endocrine regulators of physiological functions and geroprotectors: epigenetic regulatory mechanisms, Usp. Sovrem. Biol., 2017, vol. 137, no. 1, pp. 84–99.
Madaeva, I.M., Petrova, V.A., Kolesnikova, L.I., and Shevyrtalova, O.N., Obstructive sleep apnea/hypopnea syndrome and lipid peroxidation, Pul’monologiya, 2009, no. 2, pp. 65–69.
Nikitin, K.D., Heat shock proteins: biological functions and prospective use, Klin. Onkogematol. Fundam. Issled. Klin. Prakt., 2008, vol. 1, no. 2, pp. 125–130.
Pastukhov, Yu.F., Ekimova, I.V., Khudik, K.A., and Guzhova, I.V., Protein 70 kDa in the control of sleep and thermoregulation, J. Evol. Biochem. Physiol., 2008, vol. 44, no. 1, pp. 74–81.
Pastukhov, Yu.F., Khudik, K.A., and Ekimova, I.V., Role of chaperones in the regulation and recovery of physiological functions, Ross. Fiziol. Zh. im. I.M. Sechenova, 2010, vol. 96, no. 7, pp. 708–725.
Pastukhov, Yu.F., Plaksina, D.V., Lapshina, K.V., et al., Exogenous protein HSP70 blocks neurodegeneration in the rat model of the clinical stage of Parkinson’s disease, Dokl. Biol. Sci., 2014, vol. 457, no. 1, pp. 225–227.
Pastukhov, Yu.F., Simonova, V.V., Guzeev, M.A., et al., Chaperon HSP70 is involved into molecular mechanisms of regulation of slow sleep, Dokl. Ross. Akad. Nauk, 2015, vol. 461, no. 2, p. 228.
Pastukhov, Yu.F., Slow-wave sleep and molecular chaperones, J. Evol. Biochem. Physiol., 2016, vol. 52, no. 1, pp. 87–101.
Khavinson, V.Kh., Lin’kova, N.S., Trofimov, A.V., et al., Morphofunctional principles of peptide regulation of aging, Usp. Sovrem. Biol., 2011, vol. 131, no. 2, p. 115.
Khavinson, V.Kh., A single mechanism of peptide regulation of gene expression and protein synthesis in living nature, Vestn. Vosstanov. Med., 2017, no. 1, pp. 60–62.
Chebotareva, N.V., Bobkova, I.N., Neprintseva, N.I., and Kozlovskaya, L.V., Clinical determination of serum and urinary indices of HSP70 in patients with chronic glomerulonephritis, Al’m. Klin. Med., 2014, no. 30, pp. 18–24.
Anisimov, V.N. and Khavinson, V.Kh., Peptide bioregulation of aging: results and prospects, Biogerontology, 2010, vol. 11, p. 139.
Asea, A., Rehli, M., Kabingu, E., et al., Novel signal transduction pathway utilized by extracellular HSP70. Role of Toll-like receptor (TLR) 2 and TLR4, J. Biol. Chem., 2002, vol. 277, no. 17, pp. 15028–15034.
Barral, J.M., Broadley, S.A., Schaffar, G., and Hartl, F.U., Roles of molecular chaperones in protein misfolding diseases, Semin. Cell Dev. Biol., 2004, vol. 15, pp. 17–29.
Bianchi, A., Moulin, D., Hupont, S., et al., Oxidative stress-induced expression of HSP70 contributes to the inhibitory effect of 15d-PGJ2 on inducible prostaglandin pathway in chondrocytes, Free Radicals Biol. Med., 2014, vol. 76, pp. 114–126. https://doi.org/10.1016/j.freeradbiomed.2014.07.028
Calabrese, V.I., Butterfield, D.A., Scapagnini, G., et al., Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity, Antioxid. Redox Signaling, 2006, vol. 8, nos. 3–4, pp. 444–477. https://doi.org/10.1089/ars.2006.8.444
Calabrese, V.I., Cornelius, C., Dinkova-Kostova, A.T., and Calabrese, E.J., Vitagenes, cellular stress response, and acetylcarnitine: relevance to hormesis, Biofactors, 2009, vol. 35, no. 2, pp. 146–160. https://doi.org/10.1002/biof.22
Campanella, C., Pace, A., Bavisotto, C.C., et al., Heat shock proteins in Alzheimer’s disease: role and targeting, Int. J. Mol. Sci., 2018, vol. 19, no. 9, p. 2603. https://doi.org/10.3390/ijms19092603
Cedenho, A.P., Lima, S.B., Cenedeze, M.A., et al., Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa, Hum. Reprod., 2006, vol. 21, no. 7, pp. 1791–1794. https://doi.org/10.1093/humrep/del055
Correa, S.G., Maccioni, M., Rivero, V.E., et al., Cytokines and the immune-neuroendocrine network: what did we learn from infection and autoimmunity?, Cytokine Growth Factor Rev., 2007, vol. 18, no. 1–2, pp. 125–134.
Cunningham, T.J., Greenstein, J.I., Loewenstern, J., et al., Anti-inflammatory peptide regulates the supply of heat shock protein 70 monomers: implications for aging and age-related disease, Rejuvenation Res., 2015, vol. 18, no. 2, pp. 136–144. https://doi.org/10.1089/rej.2014.1620
Danzer, K.M., Ruf, W.P., Putcha, P., et al., Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity, FASEB J., 2011, vol. 25, pp. 326–336.
De Mena, L., Chhangani, D., Fernandez-Funez, P., and Rincon-Limas, D.E., secHsp70 as a tool to approach amyloid-β42 and other extracellular amyloids, Fly (Austin), 2017, vol. 11. № 3. 179–184. https://doi.org/10.1080/19336934.2017.1291104
Demirovic, D., De Toda, I.M., Nizard, C., and Rattan, S.I.S., Differential translocation of heat shock factor-1 after mild and severe stress to human skin fibroblasts undergoing aging in vitro, J. Cell Commun. Signaling, 2014, vol. 8, no. 4, pp. 333–339.
De Toda, M.I. and De la Fuente, M., The role of Hsp70 in oxi-inflamm-aging and its use as a potential biomarker of lifespan, Biogerontology, 2015, vol. 16, no. 6, pp. 709–721. https://doi.org/10.1007/s10522-015-9607-7
Ebrahimi Fakhari, D., Wahlster, L., and McLean, P.J., Molecular chaperones in Parkinson’s disease-present and future, J. Parkinsons Dis., 2011, vol. 1, no. 4, pp. 299–320.
Ekimova, I.V., Nitsinskaya, L.E., Romanova, I.V., et al., Exogenous protein HSP70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures, J. Neurochem., 2010, vol. 115, no. 4, pp. 1035–1044.
Guzhova, I.V., Kislyakova, K., Moskaliova, O., et al., In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance, Brain Res., 2001, vol. 914, pp. 66–73.
Hinault, M.P., Farina-Henriquez-Cuendet, A., and Goloubinoff, P., Molecular chaperones and associated cellular clearance mechanisms against toxic protein conformers in Parkinson’s disease, Neurodegener. Dis., 2011, vol. 8, pp. 397–412.
Jones, D.R., Moussaud, S., and McLean, P., Targeting heat shock proteins to modulate α-synuclein toxicity, Ther. Adv. Neurol. Dis., 2014, vol. 7, no. 1, pp. 33–51.
Kakimura, J., Kitamura, Y., Takata, K., et al., Microglial activation and amyloidbeta clearance induced by exogenous heat shock proteins, FASEB. J., 2002, vol. 16, no. 6, pp. 601–603.
Kraskovskaya, N.A., Kukanova, E.O., Lin’kova, N.S., et al., Correction to: tripeptides restore the number of neuronal spines under conditions of in vitro modeled Alzheimer’s disease, Bull. Exp. Biol. Med., 2017, vol. 163, no. 5, pp. 699–699. https://doi.org/10.1007/s10517-017-3882-z
Lazarev, V.F., Mikhaylova, E.R., Guzhova, I.V., and Margulis, B.A., Possible function of molecular chaperones in diseases caused by propagating amyloid aggregates, Front. Neurosci., 2017, vol. 11, p. 277. https://doi.org/10.3389/fnins.2017.00277
Malkus, K.A. and Ischiropoulos, H., Regional deficiencies in chaperone-mediated autophagy underlie α‑synuclein aggregation and neurodegeneration, Neurobiol. Dis., 2012, vol. 46, no. 3, pp. 732–744.
Naidoo, N., Ferber, M., Master, M., et al., Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling, J. Neurosci., 2008, vol. 28, no. 26, pp. 6539–6548.
Neef, D.W., Jaeger, A.M., and Thiele, D.J., Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases, Nat. Rev. Drug Discovery, 2011, vol. 10, no. 12, pp. 930–944.
Nixon, B., Bromfield, E.G., Dun, M.D., et al., The role of the molecular chaperone heat shock protein A2 (HSPA2) in regulating human sperm-egg recognition, Asian J. Androl., 2015, vol. 17, pp. 568–573. https://doi.org/10.4103/1008-682X.151395
Ohayon, M.M., Carskadon, M.A., Guilleminault, C., and Vitiello, M.V., Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan, Sleep, 2004, vol. 27, pp. 1255–1273.
Pastukhov, Y.F., Plaksina, D.V., Lapshina, K.V., et al., Exogenous protein hsp70 blocks neurodegeneration in the rat model of the clinical stage of Parkinson’s disease, Dokl. Biol. Sci., 2014, vol. 457, no. 1, pp. 225–227.
Pockley, A.G., Shepherd, J., and Corton, J.M., Detection of heat shock protein 79 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals, Immunol. Invest., 1998, vol. 6, no. 27, pp. 367–377.
Romão-Veiga, M., Matias, M.L., Nunes, V.R., et al., Cytokine Induction of systemic inflammation by hyaluronan and hsp70 in women with pre-eclampsia, Cytokine, 2018, vol. 105, pp. 23–31. https://doi.org/10.1016/j.cyto.2018.02.007
Sakharov, D.A., Stepanov, A.V., Shkurnikov, M.Yu., and Tonevitskii, A.G., Short-term highly intense physiological stress causes an increase in the expression of heat shock protein in human leukocytes, Bull. Exp. Biol. Med., 2009, vol. 147, no. 3, pp. 361–365.
Saghafi, N., Pourali, L., Ghavami Ghanbarabadi, V., et al., Serum heat shock protein 70 in pre-eclampsia and normal pregnancy: a systematic review and meta-analysis, Int. J. Reprod. Biomed., 2018, vol. 16, no. 1, pp. 1–8.
Salminen, A., Ojala, J., Kaarniranta, K., et al., Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer’s disease, Prog. Neurobiol., 2011, vol. 93, no. 1, pp. 99–110.
Vitenberga, Z. and Pilmane, M., Age-related lung tissue remodeling due to the local distribution of MMP-2, TIMP-2, TGF-β and Hsp70, Biotech. Histochem., 2018, vol. 12, pp. 1–10. https://doi.org/10.1080/10520295.2017.1421322
Wang, X., Chen, M., Zhou, J., and Zhang, X., HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (review), Int. J. Oncol., 2014, vol. 45, no. 1, pp. 18–30.
Wolfe, K.J. and Cyr, D.M., Amyloid in neurodegenerative diseases: friend or foe?, Semin. Cell. Dev. Biol., 2012, vol. 22, no. 5, pp. 476–481.
Zinsmaier, K.E. and Bronk, P., Molecular chaperones and the regulation of neutransmitter exocytosis, Biochem. Pharmacol., 2001, vol. 1, no. 62, pp. 1–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by I. Shipounova
Rights and permissions
About this article
Cite this article
Kurashova, N.A., Madaeva, I.M. & Kolesnikova, L.I. Expression of HSP70 Heat-Shock Proteins under Oxidative Stress. Adv Gerontol 10, 20–25 (2020). https://doi.org/10.1134/S2079057020010099
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079057020010099