Abstract
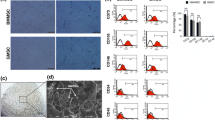
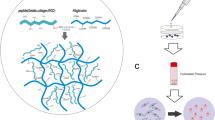
Two variants of gene-activated hydrogels based on sodium alginate containing plasmid DNA with the gene of vascular endothelial growth factor (VEGF-A) are developed. The former represented alginate hydrogel without additional components; the latter contained up to 25 wt % of octacalcium phosphate (OCP) micrograins. Alginate-based hydrogels without OCP are characterized by a honeycomb structure with the pore size of 50–200 μm and mechanical compression strength of 0.2 MPa. Addition of OCP micrograins results in filling of the polymer framework by them and an increase in the compression strength to 0.57 MPa at 16.7 wt % of OCP with a decrease to 0.49 MPa with an increase in the OCP content to 20 wt %. Both variants of gene-activated hydrogels induce reparative myogenesis in the central zone of muscle defect; a larger number of MyoG+ cells and newly formed MyH7B+ muscle fibers are identified as compared to analogous hydrogels without plasmid DNA after two weeks after surgery (p < 0.05).





Similar content being viewed by others
REFERENCES
Palmese, L.L., Thapa, R.K., Sullivan, M.O., and Kiick, K.L., Hybrid hydrogels for biomedical applications, Curr. Opin. Chem. Eng., 2019, vol. 24, pp. 143–157.
von Lospichl, B., Hemmati-Sadeghi, S., Dey, P., Dehne, T., Haag, R., Sittinger, M., Ringe, J., and Gradzielski, M., Injectable hydrogels for treatment of osteoarthritis – a rheological study, Colloids Surf., B, 2017, vol. 159, pp. 477–483.
Hussain, Z., Thu, H.E., Shuid, A.N., Katas, H., and Hussain, F., Recent advances in polymer-based wound dressings for the treatment of diabetic foot ulcer: An overview of state-of-the-art, Curr. Drug Targets, 2018, vol. 19, no. 5, pp. 527–550.
Bai, X., Gao, M., Syed, S., Zhuang, J., Xu, X., and Zhang, X.Q., Bioactive hydrogels for bone regeneration, Bioact. Mater., 2018, vol. 3, no. 4, pp. 401–417.
Deev, R.B., Drobyshev, A.Yu., and Bozo, I.Ya., Ordinary and activated osteoplastic materials, Vestn. Travmatol. Ortoped. im. N.N. Priorova, 2015, no. 1, pp. 51–69.
Odintsova, I.A., Chepurnenko, M.N., and Komarova, A.S., Myogenic satellite cells are a cambial reserve of muscle tissue, Geny Kletki, 2014, vol. 9, no. 1, pp. 6–14.
Cooper, R.N., Tajbakhsh, S., Mouly, V., et al., In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle, J. Cell Sci., 1999, vol. 112, no. 17, pp. 2895–2901.
Yin, H., Price, F., and Rudnicki, M.A., Satellite cells and the muscle stem cell niche, Physiol. Rev., 2013, vol. 93, no. 1, pp. 23–67.
Summary of Safety and Effectiveness Data (SSED). AUGMENT® Injectable. https://www.accessdata.fda. gov/cdrh_docs/pdf10/P100006S005b.pdf
Hartmann-Fritsch, F., Marino, D., and Reichmann, E., About ATMPs, SOPs and GMP: The hurdles to produce novel skin grafts for clinical use, Transfus. Med. Hemother., 2016, vol. 43, no. 5, pp. 344–352.
Gonzalez-Fernandez, T., Tierney, E.G., Cunniffe, G.M., O’Brien, F.J., and Kelly, D.J., Gene delivery of TGF-β3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering, Tissue Eng., Part A, 2016, vol. 22, nos. 9–10, pp. 776–87.
Wegman, F., Geuze, R.E., van der Helm, Y.J., Cumhur Öner, F., Dhert, W.J.A., and Alblas, J., Gene delivery of bone morphogenetic protein–2 plasmid DNA promotes bone formation in a large animal model, J. Tissue Eng. Regener. Med., 2014, vol. 8, no. 10, pp. 763–770.
Wang, P., Huang, S., Hu, Z., Yang, W., Lan, Y., Zhu, J., Hancharou, A., Guo, R., and Tang, B., In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing, Acta Biomater., 2019, vol. 100, pp. 191–201.
Song, J., Lee, M., Kim, T., Na, J., Jung, Y., Jung, G.Y., Kim, S., and Park, N., A RNA producing DNA hydrogel as a platform for a high performance RNA interference system, Nat. Commun., 2018, vol. 9, no. 1, art. ID 4331.
Villate-Beitia, I., Truong, N.F., Gallego, I., Zàrate, J., Puras, G., Pedraz, J.L., and Segura, T., Hyaluronic acid hydrogel scaffolds loaded with cationic niosomes for efficient non-viral gene delivery, RSC Adv., 2018, vol. 8, no. 56, pp. 31934–31942.
Chalanqui, M.J., Pentlavalli, S., McCrudden, C., Chambers, P., Ziminska, M., Dunne, N., and McCarthy, H.O., Influence of alginate backbone on efficacy of thermo-responsive alginate-g-P(NIPAAm) hydrogel as a vehicle for sustained and controlled gene delivery, Mater. Sci. Eng., C, 2019, vol. 95, pp. 409–421.
Grigoryan, A.S. and Shevchenko, K.G., Some possible molecular mechanisms of VEGF encoding plasmids functioning, Geny Kletki, 2011, vol. 6, no. 3, pp. 24–28.
Keeney, M., van den Beucken, J.J.J.P., van der Kraan, P.M., et al., The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF(165), Biomaterials, 2010, vol. 31, no. 10, pp. 2893–902.
Bozo, I.Ya., Deev, R.V., Zhuravlyova, M.N., Komlev, V.S., Popov, V.K., Smirnov, I.V., and Fedotov, A.Yu., Gene-activated bone substitute based on octacalcium phosphate and doped with magnesium ions, Inorg. Mater.: Appl. Res., 2018, vol. 9, pp. 70–74. https://doi.org/10.1134/S2075113318010045
Bozo, I.Ya., Maiorova, K.S., Drobyshev, A.Yu., Rozhkov, S.I., Volozhin, G.A., Eremin, I.I., Komlev, V.S., Smirnov, I.V., Rizvanov, A.A., Isaev, A.A., Popov, V.K., and Deev, R.V., Biological activity comparative evaluation of the gene-activated bone substitutes made of octacalcium phosphate and plasmid DNA carrying VEGF and SDF genes: Part 1 – In vitro, Geny Kletki, 2016, vol. 11, no. 4, pp. 34–42.
Chervyakov, Yu.V., Staroverov, I.N., Vlasenko, O.N., et al., Five-year results of treatment of patients with chronic lower limb ischemia using gene therapy, Angiol. Sosud. Khirurg., 2016, vol. 22, no. 4, pp. 38–45.
Komlev, V.S., Barinov, S.M., Bozo, I.I., et al., Bioceramics composed of octacalcium phosphate demonstrate enhanced biological behavior, ACS Appl. Mater. Interfaces, 2014, vol. 6, no. 19, pp. 16610–16620.
Bozo, I.Ya., Deev, R.V., Drobyshev, A.Yu., et al., Efficacy of gen-activated osteoplastic material based on octacalcium phosphate and plasmid DNA containing VEGF gene for critical-sized bone defects substitution, Vestn. Travmatol. Ortoped. im. N.N. Priorova, 2015, no. 1, pp. 35–42.
Wu, X., Corona, B.T., Chen, X., and Walters, T.J., A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies, BioRes. Open Access, 2012, vol. 1, no. 6, pp. 280–290.
Arsic, N., Zacchigna, S., Zentilin, L., et al., Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo, Mol. Ther., 2004, vol. 10, pp. 844–854.
ACKNOWLEDGMENTS
We are grateful to A.A. Pulin and I.I. Eremin for participation in the study.
Funding
This work was supported by the Russian Science Foundation (agreement no. 18-75-10085 on August 8, 2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Muravev
Rights and permissions
About this article
Cite this article
Bozo, I.Y., Mavlikeev, M.O., Presnyakov, E.V. et al. Gene-Activated Hydrogels Based on Sodium Alginate for Reparative Myogenesis of Skeletal Muscle. Inorg. Mater. Appl. Res. 12, 1026–1032 (2021). https://doi.org/10.1134/S2075113321040092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113321040092