Abstract
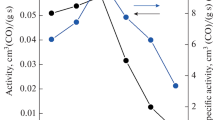
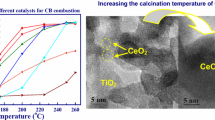
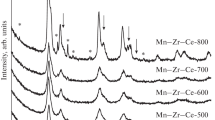
Highly dispersed MnOx–CeO2 and MnOx–ZrO2–CeO2 catalysts for the carbon monoxide oxidation reaction are synthesized. Using the XRF and the XRD methods, the formation of the Mn–Ce–O and the Mn–Zr–Ce–O solid solutions, as well as the presence of Mn2O3 and Mn3O4, is established. The specific surface area of the synthesized materials is 121 and 155 m2/g, respectively, the particle size being equal to 8–10 nm. The X-ray photoelectron (XPS) spectra is deconvolved and the relative content of the ionic forms of Mn, the lattice oxygen Oα, and the high-energy forms Oβ are determined. Upon studying the thermal stability of catalysts, it is established that the two-component systems have low thermal stability, causing the particle size to increase to 32 nm, the specific surface area to decrease to 29 m2/g, and thus the catalytic activity to deteriorate. For MnOx–ZrO2–CeO2, less significant changes take place: the particle size is 27 nm, the specific surface area is 43 m2/g, and thus its catalytic activity is higher than that for MnOx–CeO2. Investigation of the state of the components of the near-surface layer of catalysts after isothermal aging makes it possible to ascertain the change in content of ionic forms of Mn, Oα, and Oβ. It is concluded that doping of the two-component MnOx–CeO2 systems with the Zr4+ ions is expedient. The resulting ZrO2–MnOx–CeO2 solid solution is more resistant to high temperatures.







Similar content being viewed by others
REFERENCES
Trovarelli, A. and Fornasiero, P., Catalysis by Ceria and Related Materials, Singapore: World Sci., 2013.
Zou, Z.-Q., Meng, M., and Zha, Y.-Q., Surfactant-assisted synthesis, characterizations, and catalytc oxidation mechanisms of the mesoporous MnOx–CeO2 and Pd/MnOx–CeO2 catalysts used for CO and C3H8 oxidation, J. Phys. Chem. C, 2010, vol. 114, pp. 468–477.
Benalda, A., Djadoun, A., Guessis, H., and Barama, A., Effect of the preparation method on the structural and catalytic properties of MnOx–CeO2 manganese oxides, Proc. Int. Conf. Nanomaterials: Applications and Properties, 2013, vol. 2, no. 1, art. ID 01PCS105.
Shi, L., Chu, W., Qu, F., and Luo, S., Low-temperature catalytic combustion of methane over MnOx–CeO2 mixed oxide catalysts, Catal. Lett., 2007, vol. 113, no. 1, pp. 59–64.
Tang, X., Chen, J., Li, Y., Xu, Y., and Shen, W., Complete oxidation of formaldehyde over Ag/MnOx–CeO2 catalysts, Chem. Eng. J., 2006, vol. 118, pp. 119–125.
Tu, Y.-B., Luo, J.-Y, Meng, M., Wang, G., and He, J.-J., Ultrasonic-assisted synthesis of highly active catalyst Au/MnOx–CeO2 used for the preferential oxidation of CO in H2-rich stream, Int. J. Hydrogen Energy, 2009, no. 34, pp. 3743–3754.
Gongshin, Q. and Ralph, T., Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst, J. Catal., 2003, vol. 217, pp. 434–441.
Tikhomirov, K., Krocher, O., Elsener, M., and Wokaun, A., MnOx–CeO2 mixed oxides for the low-temperature oxidation of diesel soot, Appl. Catal., B, 2006, vol. 64, nos. 1–2, pp. 72–78.
Zhang, H., Wang, J., Cao, Y., Wang, Y., Gong, M., and Chen, Y., Effect of Y on improving the thermal stability of MnOx–CeO2 catalysts for diesel soot oxidation, Chin. J. Catal., 2015, vol. 36, pp. 1333–1341.
Shan, W., Ma, N., Yang, J., Dong, X., Liu, C., and Wei, L., Catalytic oxidation of soot particulates over MnOx–CeO2 oxides prepared by complexation-combustion method, J. Nat. Gas Chem., 2010, vol. 19, pp. 86–90.
Blanco, G., Cauqui, M.A., Delgado, J.J., Gaitayries, A., Perez-Omil, J.A., and Rodriquez-Izquierdo, J.M., Preparation and characterization of Ce–Mn–O composites with applications in catalytic wet oxidation processes, Surf. Interface Anal., 2004, no. 36, pp. 752–755.
Zhag, D.-Y., Murata, Y., Kishikawa, K., Ikeue, K., and Machida, M., Synthesis of large surface area MnOx–CeO2 using CTA and its catalytic activity for soot combustion, J. Ceram. Soc. Jpn., 2008, vol. 116, no. 2, pp. 230–233.
Li, J.W., Pan, K.L., Yu, S. J., Yan, S.Y., and Chang, M.B., Removal of formaldehyde over MnxCe1 – xO2 catalysts: Thermal catalytic oxidation versus ozone catalytic oxidation, J. Environ. Sci., 2014, vol. 26, pp. 2546–2553.
Xing, S., Lu, X., Ren, L., and Ma, Z., Characterization and reactivity of Mn–Ce–O composites for catalytic ozonation of antipyrine, R. Soc. Chem. Adv., 2013, vol. 74, no. 5, pp. 60279–60285.
Machida, M., Uto, M., Kurogi, D., and Kijma, T., MnOx–CeO2 binary oxides for catalytic NOx sorption at low temperatures. Sorptive removal of NOx , Chem. Mater., 2000, vol. 12, pp. 3158–3164.
Glushkova, V.B., Lapshin, A.V., Vershinin, A.A., Podzorova, L.I., and Polikanova, A.S., Phase formation of zirconia-based solid solutions synthesized from peroxides, Glass Phys. Chem., 2004, vol. 30, pp. 558–563.
Liberman, E.Y., Naumkin, A.V., Tsodikov, M.V., Mikhailichenko, A.I., Kon’kova, T.V., Grunskii, V.N., Kolesnikov, V.A., and Pereyaslavtsev, A.Y., Synthesis, structure, and properties of Au–CeO2 nanocatalyst for low-temperature oxidation of carbon monoxide, Inorg. Mater., 2017, vol. 53, no. 4, pp. 406–412.
Liberman, E.Y., Mikhailichenko, A.I., Tsodikov, M.V., Kon’kova, T.V., Morozov, A.N., and Kolesnikov, V.A., Formation particulars and thermal stability of nanodisperse systems MnOx–CeO2, Glass Ceram., 2017, vol. 74, pp. 212–215.
Xiong, Y., Tang, C., Yao, X., Zhang, L., Li, L., Wang, X., Deng, Y., Gao, F., and Dong, L., Effect of metal ions doping (M = Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3, Appl. Catal., A, 2015, vol. 495, pp. 206–216.
Tang, X., Chen, J., Huang, X., Xu, Y., and Shen, W., Pt/MnOx–CeO2 catalysts for the complete oxidation of formaldehyde at ambient temperature, Appl. Catal., B, 2008, no. 81, pp. 115–121.
Liberman, E.Yu., Kleusov, B.S., Kon’kova, T.V., and Mikhaylichenko, A.I., The catalytic activity of nanostructured MnOx–CeO2 in reaction the oxidation of carbon monoxide, Khim. Prom. Segodnya, 2011, no. 6, pp. 6–13
Smirnov, M.Y., Kalinkin, A.V., Dubkov, A.A., Vovk, E.I., Sorokin, A.M., Nizovskii, A.I., Carberry, B.P., and Bukhtiyarov, V.I., Use of the differential charging effect in XPS to determine the nature of surface compounds resulting from the interaction of a Pt/(BaCO3 + CeO2) model catalyst with SOx , Kinet. Catal., 2011, vol. 52, no. 4, pp. 595–604.
Hardacre, H., Roe, G.M., and Lambert, R.M., Structure, composition and thermal properties of cerium oxide films on platinum {111}, Surf. Sci., 1995, vol. 326, pp. 1–10.
Burroughs, P., Hamnett, A., Orchard, A.F., and Thornton, G., Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium, J. Chem. Soc., Dalton Trans., 1976, vol. 17, pp. 1686–1698.
Cao, F., Xiang, J., Su, S., Wang, P., Sun, L., Hu, S., and Lei, S., The activity and characterization of MnOx–CeO2–ZrO2/γ-Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3 , Chem. Eng. J., 2014, vol. 243, pp. 347–354.
Liu, L., Yao, Z., Liu, B., and Dong, L., Correlation of structural characteristics with catalytic performance of CuO/CexZr1 – xO2 catalysts for NO reduction by CO, J. Catal., 2010, vol. 275, pp. 45–60.
Esteves, P., Wu, Y., Dujardin, C., Dongare, M.K., and Granger, P., Ceria-zirconia mixed oxides as thermal resistant catalysts for the decomposition of nitrous oxide at high temperature, Catal. Today, 2011, vol. 176, pp. 453–457.
Wu, H., Liang, Q., Weng, D., Fan, J., and Ran, R., Synthesis of CeO2–MnOx mixed oxides and catalytic performance under oxygen-rich condition, Catal. Today, 2007, vol. 126, pp. 430–435.
Zeng, X., Huo, X., Zhu, T., Hong, X. and Sun, Y., Catalytic oxidation of NO over MnOx–CeO2 and MnOx–TiO2 catalysts, Molecules, 2016, vol. 21, pp. 1490–1452.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by I. Dikhter
Rights and permissions
About this article
Cite this article
Liberman, E.Y., Kleusov, B.S., Naumkin, A.V. et al. Thermal Stability and Catalytic Activity of the MnOx–CeO2 and the MnOx–ZrO2–CeO2 Highly Dispersed Materials in the Carbon Monoxide Oxidation Reaction. Inorg. Mater. Appl. Res. 12, 468–476 (2021). https://doi.org/10.1134/S2075113321020325
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113321020325