Abstract
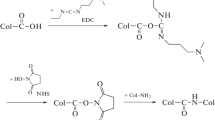
Most often, glutaraldehyde (GA) is used to increase the biostability of biological materials intended for the manufacture of implantable products or matrices for tissue-engineered constructs. However, this method has a number of side effects, including the manifestation of cytotoxicity of the final product, necessitating the search for new technological solutions. The original scleral collagen (SC) films of farm animals were obtained by the method of irrigation (sample thickness of ~150 μm), followed by drying at 370°C to a constant weight in the air. For the first group of samples, dehydrothermal treatment of SC films was carried out at a residual pressure of 10–20 mmHg and 120°C. Samples of the second group were stabilized by UV cross-linking—processing the surface with a Philips CLEO Compact lamp (16 W, 254 nm, distance from lamp to sample of 3 cm). Treatment of the SC films of third group was conducted in GA vapor at room temperature for the selected time interval (from 30 min to 18 h). The fourth group consisted of SC samples stabilized by the traditional method of cross-linking of biological tissues: treatment of aqueous 25% solution of GA with the final concentration in collagen solution from 0.0005% to 1.0% (processing time was 24 h at room temperature). The biodegradation of the samples was assessed using accelerated tests in phosphate buffer (pH 7.4, 37°C, 1 h) containing collagenase at the rate of 1 unit of enzyme per 1 mg of dry sample; the reaction was terminated after 1 h by lowering the temperature to 4°C. The films of SC (mass loss 66 ± 9%) were used as the control during the search for an optimal method of cross-linking. UV irradiation treatment weakly affected the biodegradation of the SC samples (mass loss of 55 ± 5%). The dehydrothermal treatment resulted in a linear decrease in biodegradation with increasing time of incubation, and after 18 h of treatment, the mass loss of the SC samples was practically absent. It was found that the reduction of the GA concentration in the SC solution from 1.0 to 0.001% led to a significant reduction in mass loss of samples from 20 ± 3% to 5 ± 1%. Cross-linking of the samples of SC in GA vapor also led to an increase in biostability of SC films (mass loss absent after 18 h of treatment). From four methods of SC films treatment investigated, the optimal from the point of view of increasing the biostability of collagen- based materials are dehydrothermal treatment and cross-linking by glutaraldehyde vapor.
Similar content being viewed by others
References
Sevast’anov, V.I. and Kirpichnikov, M.P., Biosovmestimye materialy (Biocompatible Materials), Moscow: Med. Inf. Agentstvo, 2011.
Schumakov, V.I. and Sevast’anov, V.I., Biopolymeric matrices for artificial organs and tissues, Zdravookhr. Med. Tekh., 2003, no. 4, pp. 30–32.
Xu, B., Chow, M.-J., and Zhang, Y., Experimental and modeling study of collagen scaffolds with the effects of cross linking and fiber alignment, Int. J. Biomater., 2011, vol. 2011. doi 10.1155/2011/172389
Goissis, G., Suzigan, S., and Parreira, D.R., Preparation and characterization of collagen-elastin matrices from blood vessels intended as small diameter vascular grafts, Artif. Organs, 2000, vol. 24, pp. 217–223.
Ehrmit, L. and Benua, O., RF Patent 2360928, 2009.
Park, S., Kim, S.H., Lim, H.G., Lim, C., and Kim, Y.J., The anti-calcification effect of dithiobispropionimidate, carbodiimide and ultraviolet irradiation crosslinking compared to glutaraldehyde in rabbit implantation models, Korean J. Thorac. Cardiovasc. Surg., 2013, vol. 46, no. 1, pp. 1–13.
Chandran, P.L., Paik, D.C., and Holmes, J.W., Structural mechanism for alteration of collagen gel mechanics by glutaraldehyde cross-linking, Connect. Tissue Res., 2012, vol. 53, no. 4, pp. 285–297, doi 10.3109/ 03008207.2011.640760
David, T.Ch., Perelman, N., Ellen, C. K., and Nimni, M., Mechanism of cross linking of proteins by glutaraldehyde III. Reaction with collagen in tissues, Connect. Tissue Res., 1985, vol. 1–3, no. 2, pp. 109–115.
Tu, R., Wang, E., Hata, C., et al., A compliant biological vascular prosthesis, Int. J. Artif. Organs, 1993, vol. 16, pp. 141–145.
Umashankar, P.R., Arun, T., and Kumari, T.V., Short duration gluteraldehyde cross-linking of decellularized bovine pericardium improves biological response, J. Biomed. Mater. Res., Part A, 2011, vol. 97, no. 3, pp. 311–320.
Grover, Ch.N., Gwynne, J.H., Pugh, N., Hamaia, S., Farndale, R.W., Best, S.M., and Ruth, E.C., Cross linking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films, Acta Biomater., 2012, vol. 8, no. 8, pp. 3080–3090.
Wess, T.J. and Orgel, J.P., Changes in collagen structure: Drying, dehydrothermal treatment and relation to long-term deterioration, Thermochim. Acta, 2000, vol. 365, nos. 1–2, pp. 119–128.
Subramanian, A. and Lin, H.-Y., Cross-linked chitosan: Its physical properties and the effects of matrix stiffness on chondrocyte cell morphology and proliferation, J. Biomed. Mater. Res., Part A, 2005, vol. 75, no. 3, pp. 742–753. doi 10.1002/jbm.a.30489
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Nemets, A.P. Pankina, V.I. Sevastianov, 2017, published in Perspektivnye Materialy, 2017, No. 3, pp. 26–32.
Rights and permissions
About this article
Cite this article
Nemets, E.A., Pankina, A.P. & Sevastianov, V.I. Comparative analysis of methods for increasing the biostability of collagen films. Inorg. Mater. Appl. Res. 8, 718–722 (2017). https://doi.org/10.1134/S2075113317050203
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113317050203