Abstract
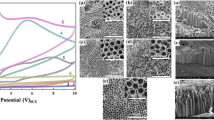
In this study, titanium dioxide nanotubes (TiO2–NTs) are prepared by using anodization method on commercial pure titanium (Ti) by applying constant voltage of 60 V to the electrolysis system for 15 min, one and two hours. Highly ordered and quality TiO2–NTs layers are formed on Ti electrode. To investigate the corrosion behavior of TiO2–NTs in 1 M KOH solution, electrochemical and surface analysis methods such as anodic and cathodic current-potential curves, electrochemical impedance spectroscopy (EIS), chronoamperometry (CA), scanning electron microscopy (SEM), atomic force microscopy (AFM) and energy dispersive X-ray spectroscopy (EDX) are used. Effect of long immersion time is also studied. Obtained results showed that the corrosion behavior of TiO2–NTs is more improved when compared to bare Ti electrode. Very stable, durable and excellent protective TiO2–NT layer is obtained at anodization time of 1 h. This oxide layer resisted the aggressive solution attack. It is suggested that this oxide layer is used in many industrial applications to reduce the corrosion of commercial pure Ti.







Similar content being viewed by others
REFERENCES
Lütjering, G., Williams, C.J., Titanium, Berlin: Springer, 2007.
Garbacz, H., Nanocrystalline Titanium, Elsevier, 2019.
Prusi, A., Arsov, L., Haran, B., and Popov, B.N., J. Electrochem. Soc., 2002, vol. 149, p. B491.
Prando, D., Brenna, A., Diamanti, M.V., Beretta, S., Bolzoni, F., Ormellese, M., and Pedeferri, M., J. Appl. Biomater. Funct. Mater., 2018, vol. 16, pp. 3–13.
Le Guéhennec, L., Soueidan, A., Layrolle, P., and Amouriq, Y., Dent. Mater., 2007, vol. 23, pp. 844–854.
Yang, Y., Ong, J.L., and Tian, J., Mater. Sci. Eng. C., 2002, vol. 20, pp. 117–124.
Vercaigne, S., Wolke, J.G.C., Naert, I., and Jansen, J.A., Biomaterials, 1998, vol. 19, pp. 1093–1099.
Montenere, A., Gnappi, G., Ferrari, F., and Cesari, M., J. Mater. Sci., 2000, vol. 35, pp. 2791–2797.
Döşlü, S.T., Mert, B.D., and Yazıcı, B., Corros. Sci., 2013, vol. 66, pp. 51–58.
Lei, B.-X., Zhang, P., Qiao, H.-K., Zheng, X.-F., et al., Electrochim. Acta, 2014, vol. 143, pp. 129–134.
Baran, E. and Yazici, B., Int. J. Hydrogen Energy, 2016, vol. 41, pp. 2498–2511.
Sun, Y. and Yan, K.-P., Int. J. Hydrogen Energy, 2014, vol. 39, pp. 11368–11375.
Alves, A.C., Wenger, F., Ponthiaux, P., Celis, J.-P., Pinto, A.M., and Rocha, L.A., Electrochim. Acta, 2017, vol. 234, pp. 16–27.
Hanawa, T., Mater. Sci. Eng. A., 1999, vol. 267, pp. 260–266.
Hanawa, T., Ukai, H., and Murakami, K., Asaoka, K., Mater. Trans.,JIM, 1995, vol. 36, pp. 438–444.
Pham, M., Maitz, M., Matz, W., Reuther, H., Richter, E., and Steiner, G., Thin Solid Films, 2000, vol. 379, pp. 50–56.
Helsen, J.A. and Breme, H.J., Metals as Biomaterials, John Wiley and Sons, 1998.
Farghali, R.A., Fekry, A.M., Ahmed, R.A., and Elhakim, H.K.A., Int. J. Biol. Macromol., 2015, vol. 79, pp. 787–799.
Jiang, W., Cui, H., and Song, Y., J. Mater. Sci., 2018, vol. 53, pp. 15130–15141.
Vera, M.L., Linardi, E., Lanzani, L., Mendez, C., Schvezon, C.E., and Ares, A.E., Mater. Corros., 2014, vol. 66, pp. 1140–1149.
Park, I.S., Woo, T.G., Jeon, W.Y., Park, H.H., Lee, M.H., Bae, T.S., and Seol, K.W., Electrochim. Acta, 2007, vol. 53, pp. 863–870.
Zhu, J. and Cui, Y., Nat. Mater., 2010, vol. 9, pp. 183–184.
Allam, N.K., Poncheri, A.J., and El-Sayed, M.A., ACS Nano, 2011, vol. 5, pp. 5056–5066.
Chen, H.M., Chen, C.K., Chen, C.-J., Cheng, L.-C., Wu, P.C., Cheng, B.H., Ho, Y.Z., Tseng, M.L, Hsu, Y.-Y., Chan, T.-S., Lee, J.-F., Liu, R.-S., and Tsai, D.P., ACS Nano, 2012, vol. 6, pp. 7362–7372.
Tsai, C.-H., Fei, P.-H., and Wu, W.-C., Electrochim. Acta, 2015, vol. 165, pp. 356–364.
Prusi, A.R. and Arsov, L.D., Corros. Sci., 1992, vol. 33, pp. 153–164.
Diamanti, M.V., Bolzoni, F., Ormellese, M., Pérez-Rosales, E.A., and Pedeferri, M.P., Corros. Eng. Sci. Technol., 2010, vol. 45, pp. 428–434.
Vetter, K.J., Electrochemical Kinetics: Theoretical and Experimental Aspects, New York: Elsevier, 1967.
Shah, U.H., Rahman, Z., Deen, K.M., Asgar, H., Shabib, I., and Haider, W., J. Appl. Electrochem., 2017, vol. 47, pp. 1147–1159.
Diao, R., Int. J. Electrochem. Sci., 2018, vol. 13, pp. 7765–7777.
González, J.E. and Mirza-Rosca, J., J. Electroanal. Chem., 1999, vol. 471, pp. 109–115.
Pan, J., Thierry, D., and Leygraf, C., Electrochim. Acta, 1996, vol. 41, pp. 1143–1153.
Souto, M.R., Laz, M.M., and Reis, L.R., Biomaterials, 2003, vol. 24, pp. 4213–4221.
Venugopalan, R., Weimer, J.J., George, M.A., and Lucas, L.C., Biomaterials, 2000, vol. 21, pp. 1669–1677.
de Assis, S.L., Wolynec, S., and Costa, I., Electrochim. Acta, 2006, vol. 51, pp. 1815–1819.
Richter, F., Schiller, C.-A., and Wagner, N., Electrochemical Applications, Kronach: Zahner-elektrik, 2002.
Jaeggi, C., Kern, P., Michler, J., Zehnder, T., and Siegenthaleri, H., Surf. Coat. Technol., 2005, vol. 200, pp. 1913–1919.
Mathis, A., Rocca, E., Veys-Renaux, D., and Tardelli, J., Electrochim. Acta, 2016, vol. 202, pp. 253–261.
Milošev, I., Žerjav, G., Calderon Moreno, J.M., and Popa, M., Electrochim. Acta, 2013, vol. 99, pp. 176–189.
Cui, W.F., Jin, L., and Zhou, L., Mater. Sci. Eng., C., 2013, vol. 33, pp. 3775–3779.
Baran, E. and Yazıcı, B., Appl. Surf. Sci., 2015, vol. 357, pp. 2206–2216.
Yasuda, K. and Schmuki, P., Electrochim. Acta, 2007, vol. 52, pp. 4053–4061.
Hlinka, J., Lasek, S., and Faisal, N., Acta Metall. Slovaca, 2017, vol. 23, pp. 270–275.
Zhang, X. and Wu, L., Ionics (Kiel), 2018, vol. 24, pp. 2905–2913.
ACKNOWLEDGMENTS
The authors are greatly thankful to Bingöl University Central Laboratory for SEM and AFM micrographs and Prof. Dr. Ramazan Solmaz and Asist. Prof. Dr. Ece Altunbaş Şahin from Bingöl University.
Funding
This study has been financially supported by the TÜBİTAK (Project no. 118Z658) and the authors are thankful to TÜBİTAK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uzal, H., Döner, A. Corrosion Behavior of Titanium Dioxide Nanotubes in Alkaline Solution. Prot Met Phys Chem Surf 56, 311–319 (2020). https://doi.org/10.1134/S207020512002029X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207020512002029X