Abstract
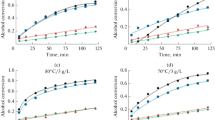
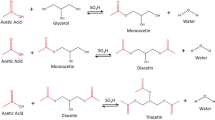
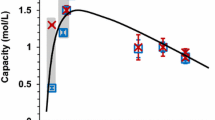
The synthesis of glycerol carbonate from glycerol and dimethyl carbonate when using strongly basic styrene–divinylbenzene anion-exchange resins Dowex 1 × 2, Dowex 1 × 4, and Dowex 1 × 8 in the OH-form is studied. The resins are characterized by different degrees of crosslinking of their polystyrene matrices (the contents of divinylbenzene are 2, 4, and 8 wt. %, respectively). Synthesis is performed at 90–105°C, and the molar ratio of dimethyl carbonate to glycerol is 2 : 1. The yield of glycerol carbonate is shown to depend on the degree of crosslinking of the anion-exchange resin, since it falls as the degree of crosslinking rises. The highest degree of the conversion of glycerol (95%) and its selectivity toward glycerol carbonate (45.5%) are observed when using Dowex 1 × 2 and the reaction proceeds at 105°C for a period of 5 h. Advantages of considered systems over other anion- and cation-exchange resins proposed in the literature are noted.







Similar content being viewed by others
REFERENCES
Ochoa-Gómez, J.R., Gómez-Jiménez-Aberasturi, O., Ramírez-López, C., and Belsué, M., Org. Process Res. Dev., 2012, vol. 16, pp. 389–399.
Transparency market research. Glycerol carbonate market. www.transparencymarketresearch.com/glycerol-carbonate-market.html. Cited April 19, 2022.
Meng, X., Wan, J., Liu, Y., Wang, X., Zhang, J., Wang, F., Zhang, J., Zheng, F., Kan, J., and Wu, G., Chem. Ind. Eng. Prog., 2020, vol. 39, pp. 3739–3749.
Teng, W.K., Ngoh, G.C., Yusoff, R., and Aroua, M.K., Energy Convers. Manage., 2014, vol. 88, pp. 484–497.
Wang, X., Zhang, P., Cui, P., Cheng, W., and Zhang, S., Chin. J. Chem. Eng., 2017, vol. 25, pp. 1182–1186.
Nogueira, D.O., de Souza, SP., Leão, R.A.C., Miranda, L.S.M., and de Souza, R.O.M.A., RSC Adv., 2015, vol. 5, pp. 20945–20950.
Ochoa-Gómez, J.R., Gómez-Jiménez-Aberasturi, O., Maestro-Madurga, B., Pesquera-Rodríguez, A., Ramírez-López, C., Lorenzo-Ibarreta, L., Torrecilla-Soria, J., and Villarán-Velasco, M.C., Appl. Catal., A, 2009, vol. 366, pp. 315–324.
Bai, R., Wang, Y., Wang, S., Mei, F., Li, T., and Li, G., Fuel Process. Technol., 2013, vol. 106, pp. 209–214.
Rokicki, G., Rakoczy, P., Parzuchowski, P., and Sobiecki, M., Green Chem., 2005, vol. 7, pp. 529–539.
Li, J. and Wang, T., J. Chem. Thermodyn., 2011, vol. 43, pp. 731–736.
Malyaadri, M., Jagadeeswaraiah, K., Sai Prasad, P.S., and Lingaiah, N., Appl. Catal., A, 2011, vol. 401, pp. 153–157.
Algoufi, Y.T., Akpan, U.G., Kabir, G., Asif, M., and Hameed, B.H., Energy Convers. Manage, 2017, vol. 138, pp. 183–1929.
Kumar, A., Iwatani, K., Nishimura, S., Takagaki, A., and Ebitani, K., Catal. Today, 2012, vol. 185, pp. 241–246.
Takagaki, A., Iwatani, K., Nishimura, S., and Ebitani, K., Green Chem., 2010, vol. 12, pp. 578–581.
Bai, R., Wang, S., Mei, F., Li, T., and Li, G., J. Ind. Eng. Chem., 2011, vol. 17, pp. 777–781.
Jarvis, I., Totland, M.M., and Jarvis, K.E., Analyst, 1997, vol. 122, pp. 19–26.
Kaya, A., Kud, H., Shrahashi, J., and Suzuki, S., J. Nucl. Sci. Technol., 1967, vol. 4, pp. 289–292.
Stoliker, D.L., Kaviani, N., Kent, D.B., and Davis, J.A., Geochem. Trans., 2013, vol. 14, pp. 1–9.
Hatch, J.A. and Dillon, H.B., Ind. Eng. Chem. Process Des. Dev., 1963, vol. 2, pp. 253–263.
Suh, J. and Park, C., Bull. Korean Chem. Soc., 1991, vol. 12, pp. 113–115.
Marrodan, C.M., Beboraerti, D., Liguoria, F., and Barbaro, P., Catal. Sci. Technol., 2012, vol. 2, pp. 2279–2290.
DOWEX™ fine mesh spherical ion exchange resins. https://www.lenntech.com/Data-sheets/Dowex-1x8-100-200-L.pdf. Cited April 18, 2022.
Nesterov, Yu.V., Ionity i ionoobmen. Sorbtsionnaya tekhnologiya pri dobyche urana i drugikh metallov metodom podzemnogo vyshchelachivaniya (Ionites and Ion Exchange. Sorption Technology in the Production of Uranium and Other Metals by Underground Leaching), Moscow: Vneshtorgizdat, 2007.
Abdullaev, M.G., Vestn. Dagestan. Gos. Univ., Estestv. Nauki, 2017, vol. 32, no. 1, pp. 54–60.
Tsuru, T., Sasaki, A., Kanezashi, M., and Yoshioka, T., AIChE J., 2011, vol. 57, pp. 2079–2089.
Ngai, K.L., Lunkenheimer, P., and Loidl, A., Phys. Chem. Chem. Phys., 2020, vol. 22, pp. 507–511.
Funding
This work was supported by the RF Ministry of Higher Education and Science as part of a State Task for the Boreskov Institute of Catalysis, project no. AAAA-A21-121011390055-8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Kukharuk
Rights and permissions
About this article
Cite this article
Shvydko, A.V., Prihod’ko, S.A. & Timofeeva, M.N. Synthesis of Glycerol Carbonate from Glycerol and Dimethyl Carbonate Using Strongly Basic Anion-Exchange Styrene–Divinylbenzene Dowex Resins. Catal. Ind. 14, 181–188 (2022). https://doi.org/10.1134/S2070050422020088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050422020088