Abstract
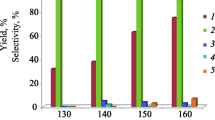
Development of catalysts based on modified zeolites with a microporous or micro/mesoporous structure and on microporous metallosilicates is aimed at creating high-selectivity method of obtaining pyridine and alkylpyridines. In this study, pyridine and methylpyridines have been synthesized for the first time via the heterogeneous catalytic reaction of ethanol with formaldehyde and ammonia catalyzed by the microporous zeolites Y, Beta, ZSM-12, and ZSM-5 in the H-form and by a granular zeolite Y with a combined, micro-meso-macroporous structure (HY-MMM). The latter zeolite is particularly effective in the synthesis of picolines, affording a picoline selectivity of 46–63% at an ethanol conversion of 70–80%. Among the microporous catalysts, the most active ones are the highly decationized zeolites H-Y and H-Beta. The major products of the reaction occurring over H-Beta and H-ZSM-5 are pyridine (up to 50%) and picolines (up to 40%), and the main products of the same reaction carried out over H-Y and H-ZSM-12 are picolines (45–52%) and lutidines (19–25%). For zeolite H-Y-MMM, the ethanol conversion and the composition of pyridines depend on the reaction conditions.
Similar content being viewed by others
References
Golunski, S.E. and Jackson, D., Appl. Catal., 1986, vol. 23, no. 1, pp. 1–14.
Henry, G.D., Tetrahedron, 2004, vol. 60, no. 29, pp. 6043–6061.
Scriven, E.F.V., Toomey, J.E., and Murugan, R., in Kirk-Othmer Encyclopedia of Chemical Technology, Kroschwitz, J.I. and Howe-Grant, M., Eds., New York: Wiley, 1996, vol. 20, pp. 641–679.
Higasio, Y.S. and Shoji, T., Appl. Catal., A, 2001, vol. 221, nos. 1–2, pp. 197–207.
Weissermel, K. and Arpe, H.J., Industrial Organic Chemistry, New York: Wiley, 2008.
Pyridines—IHS Chemical Economics Handbook. http://www.ihs.com/products/chemical/planning/ceh/pyridines.aspx. Cited September 21, 2015.
Krishna Mohan Kandepi, V.V. and Narender, N., Catal. Sci. Technol., 2012, vol. 2, pp. 471–487.
Reddy, K.S.K., Sreedhar, I. and Raghavan, K.V., Appl. Catal., A, 2008, vol. 339, no. 1, pp. 15–20.
Reddy, K.S.K., Sreedhar, I., Venkateshwar, S., and Raghavan, K.V., Catal. Lett., 2008, vol. 125, no. 1, pp. 110–115.
Reddy, K.S.K., Sreedhar, I., and Raghavan, K.V., Can. J. Chem. Eng., 2011, vol. 89, no. 4, pp. 854–863.
EP Patent 0837849, 2002.
US Patent 7365204, 2008.
Shimizu, S., Abe, N., Iguchi, A., Dohba, M., Sato, H., and Hirose, K., Microporous Mesoporous Mater., 1998, vol. 21, nos. 4–6, pp. 447–451.
EP Patent 1167352, 2006.
US Patent 6281362, 2001.
Van der Gaag, F.J., Louter, F., Oudejans, J.C. and van Bekkum, H., Appl. Catal., 1986, vol. 26, pp. 191–201.
Rama Rao, A.V., Kulkarni, S.J., Ramachandra Rao, R., and Subrahmanyanm, M., Appl. Catal., A, 1994, vol. 111, no. 2, pp. L101–L108.
Kulkarni, S.J., Ramachandra Rao, R., Subrahmanyanm, M. and Rama Rao, A.V., Appl. Catal., A, 1994, vol. 113, no. 1, pp. 1–7.
Khazipova, A.N., Pavlova, I.N., Grigor’eva, N.G., Kutepov, B.I., Pavlov, M.L., and Basimova, R.A., Khim. Tekhnol., 2012, no. 1, pp. 5–9.
RF Patent 2 456 238, 2012.
Khazipova, A.N., Kutepov, B.I., Pavlov, M.L., Grigor’eva, N.G., Shestopal, Ya.L., Pashkina, A.N., and Travkin, E.A., Russ. J. Appl. Chem., 2007, vol. 80, no. 11, pp. 1841–1844.
Yushchenko, V.V., Zh. Fiz. Khim., 1997, vol. 71, no. 4, pp. 628–632.
Plachenov, T.G. and Kolosentsev, S.D., Porometriya (Porosimetry), Moscow: Khimiya, 1988.
Kel’tsev, N.V., Osnovy adsorbtsionnoi tekhniki (Fundamentals of Adsorption Technique), Moscow: Khimiya, 1975.
Basimova, R.A., Liquid-phase disproportionation of diethylbenzenes and benzene to ethylbenzene over zeolite catalysts, Cand. Sci. (Chem.) Dissertation, Ufa: Inst. Petrochem. Catal. RAS, 2009.
Baerlocher, C., McCusker, L.B., and Olson, D.H., Atlas of Zeolite Framework Types, Amsterdam: Elsevier, 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.G. Grigor’eva, N.A. Filippova, A.N. Khazipova, O.S. Travkina, B.I. Kutepov, 2015, published in Kataliz v Promyshlennosti.
Rights and permissions
About this article
Cite this article
Grigor’eva, N.G., Filippova, N.A., Khazipova, A.N. et al. Zeolite catalysts with various porous structures in the synthesis of pyridines. Catal. Ind. 7, 287–292 (2015). https://doi.org/10.1134/S207005041504008X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207005041504008X