Abstract
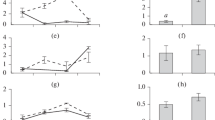
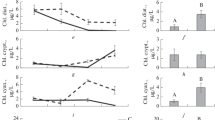
The efficiency of transfer of essential substances (carbon, phosphorus, nitrogen, and fatty acids (FAs), including polyunsaturated fatty acids (PUFAs)) from phytoplankton to zooplankton in three habitats (pelagic zone and vegetated and unvegetated littoral areas) in mesotrophic Lake Obsterno (Belarus) has been assessed. The efficiency of transfer of substances was calculated as the ratio of their secondary production to primary production per water volume unit and biomass unit. The efficiency per water volume unit can characterize the efficiency of transferring substances in a lake, while the efficiency per biomass unit indicates the ability of zooplankton to accumulate essential substances consumed with the food resources in biomass, i.e. it characterizes the quality of zooplankton as a resource for higher trophic levels. The efficiency of PUFA transfer was lower than the carbon transfer efficiency. On the contrary, the accumulation of nutrients, especially phosphorus, was more effective when compared with the carbon accumulation rate. This indicates that zooplankton serve as a sink for nutrients which are transferred up the trophic webs. The plankton communities in the pelagic zone of mesotrophic lake were found to be more effective in transferring the substances from the primary producers to the consumers than the littoral communities were.




Similar content being viewed by others
REFERENCES
Alimov, A.F., Vvedenie v produktsionnuyu gidrobiologiyu (An Introduction to Production Hydrobiology), Leningrad: Gidrometeoizdat, 1989.
Balushkina, E.V. and Winberg, G.G., The relationship between the length and body weight of planktonic crustaceans and rotifers, in Ekologo-fiziologicheskie osnovy izucheniya vodnykh ekosistem (Ecological and Physiological Principles of the Study of Aquatic Ecosystems), Leningrad: Nauka, 1979, p. 169.
Becker, C. and Boersma, M., Differential effects of phosphorus and fatty acids on daphnia magna growth and reproduction, Limnol. Oceanogr., 2005, vol. 50, p. 388.
Blue Book of Belarus, Minsk: Bel. En., 1994.
Boersma, M., Schops, C., and McCauley, E., Nutritional quality of seston for the freshwater herbivore Daphnia galeatahyalina: biochemical versus mineral limitation, Oecologia, 2001, vol. 129, p. 342.
Buseva, Zh. and Pljuta, M., Feeding of YOY fish in littoral zone of shallow lake, Proc. Natl. Acad. Sci. Bel., 2015, vol. 59, no. 3, p. 71.
DeMott, W.R., Utilization of a cyanobacterium and a phosphorus-deficient green alga as a complementary resource by daphnids, Ecology, 1998, vol. 79, p. 2463.
DeMott, W.R., Gulati, R.D., and Siewertsen, K., Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna, Limnol., Oceanogr., 1998, vol. 43, p. 1147.
Dickman, E.M., Newell, J.M., Gonzalez, M.J., and Vanni, M.J., Light, nutrients, and food chain length constrain planktonic energy transfer efficiency across multiple trophic levels, Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, p. 18408. https://doi.org/10.1073/pnas.0805566105
Elser, J.J., Kyle, M., Frost, P., et al., Effects of light and nutrients on plankton stoichiometry and biomass in a P-limited lake, Hydrobiologia, 2002, vol. 481, p. 101.
Feller, S.E., Acyl chain conformations in phospholipid bilayers: a comparative study of docosahexaenoic acid and saturated fatty acids, Chem. Phys. Lipids, 2008, vol. 153, p. 76. https://doi.org/10.1016/j.chemphyslip.2008.02.013
Gladyshev, M.I., Kolmakov, V.I., Dubovskaya, O.P., and Ivanova, E.A., The microalgal food spectrum of Daphnia longispina during the algal bloom of an eutrophic water body, Dokl. Biol. Sci., 2000, vol. 371, p. 179.
Gladyshev, M.I., Sushchik, N.N., Dubovskaya, O.P., et al., Influence of sestonic elemental and essential fatty acid contents in a eutrophic reservoir in Siberia on population growth of Daphnia (longispina group), J. Plankton Res., 2006, vol. 28, no. 10, p. 907. https://doi.org/10.1093/plankt/fbl028
Gladyshev, M.I., Sushchik, N.N., Kolmakova, A.A., et al., Seasonal correlations of elemental and v-3 PUFA composition of seston and dominant phytoplankton species in a eutrophic Siberian reservoir, Aquat. Ecol., 2007, vol. 41, p. 9. https://doi.org/10.1007/s10452-006-9040-8
Gladyshev, M.I., Sushchik, N.N., Anishchenko, O.V., et al., Efficiency of transfer of essential polyunsaturated fatty acids versus organic carbon from producers to consumers in a eutrophic reservoir, Oecologia, 2011, vol. 165, p. 521. https://doi.org/10.1007/s00442-010-1843-6
Glencross, B.E., Exploring the nutritional demand for essential fatty acids by aquaculture species, Rev. Aquacult., 2009, vol. 1, no. 2, pp. 71–124. https://doi.org/10.1111/j.1753-5131.2009.01006.x
Gulati, R.D. and DeMott, W.R., The role of food quality for zooplankton: remarks on the state -of-the-art, perspectives and priorities, Freshwater Biol., 1997, vol. 38, no. 3, p. 753. https://doi.org/10.1046/j.1365-2427.1997.00275.x
Kalachova, G.S., Gladyshev, M.I., Sushchik, N.N., and Makhutova, O.N., Water moss as a food item of the zoobenthos in the Yenisei River, Centr. Eur. J. Biol., 2011, vol. 6, p. 236. https://doi.org/10.2478/s11535-010-0115-0
Karpowicz, M., Feniova, I., Gladyshev, M.I., et al., The stoichiometric ratios (C:N:P) in a pelagic food web under experimental conditions, Limnologica, 2019, vol. 77, p. 125690. https://doi.org/10.1016/j.limno.2019.125690
Lacroix, G., Biomass and production of plankton in shallow and deep lakes: are there general patterns? Ann. Limnol., 1999, vol. 35, no. 2, p. 111.
Lindeman, R.L., The trophic-dynamic aspect of ecology, Ecology, 1942 vol. 23, p. 399.
Loladze, I. and Elser, J.J., The origins of the red-field nitrogen-to-phosphorus ratio are in a homoeostatic protein-to-rRNA ratio, Ecol. Lett., 2011, vol. 14, p. 244. https://doi.org/10.1111/j.1461-0248.2010.01577.x
Mikheeva, T.M., Methods of quantitative estimates of nanophytoplankton (review), Hydrobiol. J., 1989, vol. 25, p. 3.
Mikheeva, T.M., Algal flora of Belarus, in Taksonomicheskii katalog (Taxonomic Catalogue), Minsk: Bel. Gos. Univ., 1999.
Mikheeva, T.M., Assessment of the production capabilities of a unit of phytoplankton biomass, in Biologicheskaya produktivnost' evtrofnogo ozera (Biological Productivity of an Eutrophic Lake), Moscow: Nauka, 1970, p. 50.
Müller-Navarra, D.C., Biochemical versus mineral limitation in Daphnia, Limnol. Oceanogr., 1995, vol. 40, p. 1209.
Murphy, J. and Riley, J.P., A modified single solution method for the determination of phosphate in natural waters, Anal. Chim. Acta, 1962, vol. 27, p. 31.
Schmitz, G. and Ecker, J., The opposing effects of n-3 and n-6 fatty acids, Prog. Lipid Res., 2008, vol. 47, p. 147. https://doi.org/10.1016/j.plipres.2007.12.004
Slobodkin, L.B., On the inconstancy of ecological efficiency and the form of ecological theories, in Growth by Intussusception: Ecological Essays in Honor of G.E. Hutchinson, New Haven: Trans. Conn. Acad. Arts Sci., 1972, vol. 44, p. 291.
Sommer, U. and Sommer, F., Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton, Oecologia, 2006, vol. 147, p. 183. https://doi.org/10.1007/s00442-005-0320-0
Sommer, U., Gliwicz, Z.M., Lampert, W., and Duncan, A., The peg-model of a seasonal succession of planktonic events in fresh waters, Arch. Hydrobiol., 1986, vol. 106, p. 433.
Sterner, R.W., Daphnia growth on varying quality of Scenedesmus: mineral limitation of zooplankton, Ecology, 1993, vol. 74, p. 2351.
Sterner, R.W., Modelling interactions of food quality and quantity in homeostatic consumers, Freshwater Biol., 1997, vol. 38, pp. 473–481. https://doi.org/10.1046/j.1365-2427.1997.00234.x
Sterner, R.W. and Elser, J.J., Ecological Stoichiometry: the Biology of Elements from Molecules to the Biosphere, New York: Princeton Univ. Press, 2002.
Sterner, R.W. and Schulz, K.L., Zooplankton nutrition: recent progress and a reality check, Aquat. Ecol., 1998, vol. 32, p. 261.
Sterner, R.W., Clasen, J., Lampert, W., and Weisse, T., Carbon: phosphorus stoichiometry and food chain production, Ecol. Lett., 1998, vol. 1, p. 146. https://doi.org/10.1046/j.1461-0248.1998.00030.x
Stockwell, J.D. and Johannsson, O.E., Temperature-dependent allometric models to estimate zooplankton production in temperate freshwater lakes, Can. J. Fish. Aquat. Sci., 1997, no. 54, p. 2350. https://doi.org/10.1139/f97-141
Taipale, S.J., Kainz, M.J., and Brett, M.T., Diet-switching experiments show rapid accumulation and preferential retention of highly unsaturated fatty acids in Daphnia, Oikos, 2011, vol. 120, p. 1674. https://doi.org/10.1111/j.1600-0706.2011.19415.x
Wacker, A. and Von Elert, E., Polyunsaturated fatty acids: evidence for non-substitutable biochemical resources in Daphnia galeata, Ecology, 2001, vol. 82, p. 2507.
Wagner, N.D., Lankadurai, B.P., Simpson, M.J., et al., Metabolomic differentiation of nutritional stress in an aquatic invertebrate, Physiol. Biochem. Zool., 2015, vol. 88, p. 43. https://doi.org/10.1086/679637
Wassell, S.R. and Stillwell, W., docosahexaenoic acid domains: the ultimate non-raft membrane domain, Chem. Phys. Lipids, 2008, vol. 153, p. 57. https://doi.org/10.1016/j.chemphyslip.2008.02.010
Winberg, G.G. and Lavrenteva, G.M., Guidelines for the collection and processing of materials for hydrobiological studies in freshwater bodies, in Fitoplankton i ego produktsiya (Phytoplankton and Its Products), Leningrad: Gos. Nauchno-Issled. Inst. Ozern. Rechn. Rybn. Khoz., 1982, p. 1.
White, T.C.R., The Inadequate Environment, Berlin: Springer, 1993.
Yacobi, Y.Z. and Zohary, T., Carbon: chlorophyll a ratio, assimilation numbers and turnover times of Lake Kinneret phytoplankton, Hydrobiologia, 2010 vol. 639, p. 185. https://doi.org/10.1007/s10750-009-0023-3
Funding
The collection, treatment, and analysis of the phytoplankton and zooplankton samples were funded by the Belarusian Republican Foundation for Fundamental Research (BRFFI), project no. B18R-004; seston collection and treatment for the elemental composition were performed as part of BRFFI grant no. B17-037. The interpretation of results, literature reviews, and manuscript preparation for publishing were supported by the Russian Foundation for Basic Research, project no. 18-54-00002Bel_a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interests.
Statement of welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by O. Zhiryakova
Abbreviations: Bphyto—phytoplankton biomass; Bzoo—raw zooplankton biomass; SPzoo—secondary production of crustaceans; DHA—dokozahexaenoic acids; FA—fatty acids; PUFA—polyunsaturated fatty acids; PP—primary production; EPA—ecosapentaenoic acid.
Rights and permissions
About this article
Cite this article
Buseva, Z.F., Gladyshev, M.I., Sushchik, N.N. et al. Efficiency of Transfer of Essential Substances from Phytoplankton to Planktonic Crustaceans in Mesotrophic Lake Obsterno (Belarus). Inland Water Biol 14, 391–400 (2021). https://doi.org/10.1134/S1995082921030032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082921030032