Abstract
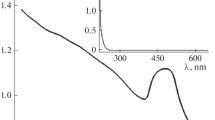
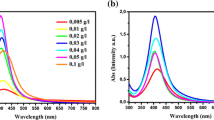
This article is devoted to studying the kinetics of formation of individual nanoparticles of noble metals and composites based on them. The effect of the concentration of the reducing agent and temperature on the rate of formation of nanoparticles of noble metals is studied. In order to characterize the morphology, phase composition, and optical properties of the synthesized nanomaterials, X-ray diffraction, transmission electron microscopy, UV–visible spectroscopy, and Raman scattering (RS) are used. It is shown that the formation of nanoparticles of noble metals and composites based on them is a three-step process consisting of an induction period, the process of nucleation, and the particle growth stage (which has an autocatalytic character). The formed nanoparticles of noble metals catalyze the further process of reduction of ions remaining in the solution. It has been confirmed that an important factor for amplifying the signal from the analyte molecules in Raman spectroscopy is the resonance absorption by nanoparticles of noble metals in a wavelength range close to the wavelength of the excitation laser.
Similar content being viewed by others
References
L. Lu, G. Burkey, I. Halaciuga, and D. V. Goia, “Core–shell gold/silver nanoparticles: synthesis and optical properties,” J. Colloid Interface Sci. 392, 90–95 (2013).
J. Kim, S.-Ch. Hong, H.-J. Jang, Y.-ju. Choi, and S. Park, “Influence of iodide ions on morphology of silver growth on gold hexagonalnanoplates,” J. Colloid Interface Sci. 389, 71–76 (2013).
Y. Okuno, K. Nishioka, A. Kiya, N. Nakashima, A. Ishibashi, and Y. Niidome, “Uniform and controllable preparation of Au–Ag core–shell nanorods using anisotropic silver shell formation on gold nanorods,” Nanoscale 2, 1489–1493 (2010).
R. Jiang, H. Chen, L. Shao, Q. Li, and J. Wang, “Unraveling the evolution and nature of the plasmons in (Au core)–(Ag shell) nanorods,” Adv. Mater. 24, OP200–OP207 (2012).
G. Zhang, J. B. Jasinski, J. L. Howell, D. Patel, D. P. Stephens, and A. M. Gobin, “Tunability and stability of gold nanoparticles obtained from chloroauric acid and sodium thiosulfate reaction,” Nanoscale Res. Lett. 7, 1–9 (2012).
E. Csapó, A. Oszkó, E. Varga, Á. Juhász, Norbert Buzás, L. Korösi, A. Majzik, I. Dékány, “Synthesis and characterization of Ag/Au alloy and core (Ag)–shell (Au) nanoparticles,” Colloids Surf. A: Physicochem. Eng. Aspects 415, 281–287 (2012).
E. H. Nanaie, A. Cooper, Sh. Sanders, and D. Zhao, “Inter-dendrimer stabilized Ag/Au alloy and core-shell nanoparticles,” Phys. Natural Sci., 1–7 (2007).
M. Gellner, B. Kustner, and S. Schlucker, “Optical properties and SERS efficiency of tunable gold/silver nanoshells,” Vibrat. Spectrosc. 50, 43–47 (2009).
Sh. Liu, G. Chen, P. N. Prasad, and M. T. Swihart, “Synthesis of monodisperse Au, Ag, and AuAg alloy nanoparticles with tunable size and surface plasmon resonance frequency,” Chem. Mater. 23, 4098–4101 (2011).
R. Contreras-Caceres, C. Dawson, P. Formanek, D. Fischer, F. Simon, A. Janke, P. Uhlmann, and M. Stamm, “Polymers as templates for Au and AuAg bimetallic nanorods: UV-Vis and surface enhanced Raman spectroscopy,” Chem. Mater. 25, 158–169 (2013).
X. Liu, A. Wang, X.-f. Yang, T. Zhang, Ch.-Y. Mou, D.-Sh. Su, and J. Li, “Synthesis of thermally stable and highly active bimetallic Au-Ag nanoparticles on inert supports,” Chem. Mater. 21, 410–418 (2009).
J. Turkevich, P. C. Stevenson, and J. Hillier, “The formation of colloidal gold,” J. Phys. Chem. 57, 670–673 (1953).
J. A. Peck, C. D. Tait, B. I. Swanson, and G. E. Brown, “Speciation of aqueous gold(III) chlorides from ultraviolet/visible absorption and Raman/resonance Raman spectroscopies,” Geochim. Cosmochim. Acta 55, 671–676 (1991).
M. Zhang, A. Zhao, H. Sun, H. Guo, D. Wang, D. Li, Z. Gan, and W. Tao, “Rapid, large-scale, sonochemical synthesis of 3D nanotextured silver microflowers as highly efficient SERS substrates,” J. Mater. Chem. 21, 18817–18824 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Eremina, D.P. Kapusta, M.O. Volodina, A.V. Sidorov, A.V. Grigorieva, E.A. Goodilin, 2015, published in Rossiiskie Nanotekhnologii, 2015, Vol. 10, Nos. 9–10.
Rights and permissions
About this article
Cite this article
Eremina, E.A., Kapusta, D.P., Volodina, M.O. et al. Investigation of kinetics of the process of formation of gold and silver nanoparticles and composites based on them. Nanotechnol Russia 10, 713–726 (2015). https://doi.org/10.1134/S1995078015050067
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078015050067