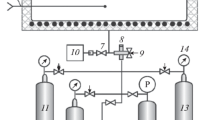
Abstract
The ignition temperatures and effective activation energies of mixtures of 5–40% H2–air over metallic Rh and stoichiometric mixtures (30–70% H2 + 70–30% C2H6 (and C2H4)) + air over metallic Rh and Pd are experimentally determined at pressures of 1 to 2 atm over the temperature range 20–300°C. It is shown that, for the investigated mixtures, metallic Rh is more effective than Pd, and the effective activation energies of ignition depend not only on the nature of the catalyst but also on the chemical nature of the hydrocarbon in the mixture. The data obtained indicate that catalytic ignition is initiated only by an exothermic surface reaction of hydrogen oxidation on the catalyst; the hydrocarbon on the surface is consumed in reactions involving intermediate products of hydrogen oxidation that do not lead to chain branching; and then combustion propagates into the volume. It is established that in an untreated reactor, the ignition temperature of the mixture of 70% H2 + 30% methane with air above the surface of palladium at a pressure of 1.75 atm is 310°C; and above the surface of rhodium, 105°C. In a reactor treated with ignition, the ignition temperature of a mixture of 70% H2 + 30% methane with air above the surface of palladium at a pressure of 1.75 atm is 270°C; and above the surface of rhodium, 62°C. The result obtained indicates the potential of using a rhodium catalyst to significantly lower the ignition temperature of fuels based on methane and hydrogen mixtures.




Similar content being viewed by others
REFERENCES
B. Lewis and G. von Elbe, Combustion, Explosions and Flame in Gases (Academic, New York, 1987).
K. Persson, L. D. Pfefferle, W. Schwartz, A. Ersson, and S. G. Jaras, Appl. Catal. B: Environ. 74, 242 (2007).
A. Fernández, G. M. Arzac, U. F. Vogt, et al., Appl. Catal. 180, 336.
E.-S. Cho and S. H. Chung, J. Mech. Sci. Technol. 23, 650 (2009).
H. Razali, K. Sopian, and S. Mat, ARPN J. Eng. Appl. Sci. 10, 7780 (2015).
A. A. Borisov, N. M. Rubtsov, G. I. Skachkov, and K. Ya. Troshin, Russ. J. Phys. Chem. B 6, 517 (2012).
K. Ya. Troshin, N. M. Rubtsov, G. I. Tsvetkov, and V. I. Chernysh, Russ. J. Phys. Chem. B 16, 39 (2022).
N. M. Rubtsov, A. N. Vinogradov, A. P. Kalinin, A. I. Rodionov, I. D. Rodionov, K. Ya. Troshin, G. I. Tsvetkov, and V. I. Chernysh, Russ. J. Phys. Chem. B 13, 305 (2019).
N. M. Rubtsov, G. I. Tsvetkov, V. I. Chernysh, and K. Ya. Troshin, Combust. Flame 218, 179 (2020).
A. A. Borisov, V. G. Knorre, E. L. Kudryashova, G. I. Skachkov, and K. Ya. Troshin, Khim. Fiz. 17 (7), 80 (1998).
A. P. Kalinin, N. M. Rubtsov, A. N. Vinogradov, V. V. Egorov, N. A. Matveeva, A. I. Rodionov, A. Yu. Sazonov, K. Ya. Troshin, G. I. Tsvetkov, and V. I. Chernysh, Russ. J. Phys. Chem. B 14, 413 (2020).
Engineering ToolBox, Coefficients of Linear Thermal Expansion, 2003. www.engineeringtoolbox.com/linear-expansion-coefficients-d_95.html
S. M. Repinskii, Introduction to the Chemical Physics of the Surface of Solids (Nauka, Novosibirsk, 1993) [in Russian].
N. N. Semenov, On Some Problems of Chemical Kinetics and Reactivity (Akad. Nauk SSSR, Moscow, 1958; Elsevier, Amsterdam, 1958).
I. D. Rodionov, N. M. Rubtsov, A. N. Vinogradov, A. P. Kalinin, I. D. Rodionov, K. Ya. Troshin, G. I. Tsvetkov, V. I. Chernysh, and B. S. Seplyarsky, Russ. J. Phys. Chem. B 15, 702 (2021).
Funding
In terms of studying combustion over the surface of metallic rhodium and palladium using high-speed color filming, this study was supported by the state orders AAAA-A17-117011910011-09 and ISMAN; and in terms of studying the combustion of hydrogen–methane–air mixtures, this study was supported by the state assignment nos. 0082 (registration number AAAA-A21-121011990037-8) and ISMAN (registration number AAAA-A19-119010990034-5) of the Institute of Chemical Physics of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Troshin, K.Y., Rubtsov, N.M., Tsvetkov, G.I. et al. Initiation of Hydrogen–Air Mixtures with Metallic Rh and Hydrogen–Methane/Ethane/Ethylene–Air Mixtures with Pd and Rh at Pressures of 1–2 atm. Russ. J. Phys. Chem. B 16, 693–698 (2022). https://doi.org/10.1134/S1990793122040303
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122040303