Abstract
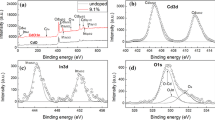
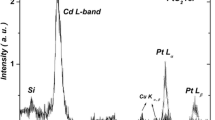
We investigate theoretically the main aspects of the formation of cadmium oxide thin films (by metal evaporation) relative to non-stoichiometry and partial pressure of oxygen. As a matter of fact, mathematical relationships are obtained from that the Cd and CdO phases coexist when the corresponding chemical reaction begins. In fact, the Cd pressure (equal to the CdO one) is found to be proportional to the oxygen absolute temperature which, in turn, equals the Cd (or CdO) absolute temperature. Moreover, the electron drift-mobility in CdO is obtained as a function of the above temperature and as a function of the oxygen partial pressure. We emphasize that chemical kinetics is studied quantitatively by taking into account that a part of cadmium does not combine with oxygen, that is, stoichiometry vanishes. This means that, initially, CdO is still amorphous. This state occurs when the partial pressure of oxygen is smaller than the so-called optimum value of pressure which, by definition, is the value of the oxygen partial pressure at which the optical transmittance of CdO is maximum. These issues are investigated (by means of theoretical considerations) accurately in consistency with experimental data.
Similar content being viewed by others
REFERENCES
C. Sravani, K. T. R. Reddy, and P. J. Reddy, Mater. Lett. 15, 356 (1993).
A. A. Dakhel, Curr. Appl. Phys. 11, 11 (2011).
F. C. Eze, Nuovo Cim. D 20, 1421 (1998).
F. C. Eze, Global J. Pure Appl. Sci. 9, 567 (2003).
F. C. Eze, Mater. Chem. Phys. 89, 205 (2005).
Y. Hames and S. E. San, Solar Energy 77, 291 (2004).
D. J. Seo, J. Korean Phys. Soc. 45, 1575 (2004).
M. A. Grado-Caffaro and M. Grado-Caffaro, Mod. Phys. Lett. B 9, 1631 (1995).
M. A. Grado-Caffaro and M. Grado-Caffaro, Mod. Phys. Lett. B 18, 1255 (2004).
M. A. Grado-Caffaro and M. Grado-Caffaro, Phys. Lett. A 372, 4858 (2008).
M. A. Grado-Caffaro and M. Grado-Caffaro, Mod. Phys. Lett. B 29, 1550206 (2015).
M. A. Grado-Caffaro and M. Grado-Caffaro, Russ. J. Phys. Chem. A 92, 2723 (2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Grado-Caffaro, M.A., Grado-Caffaro, M. On Non-stoichiometry and Partial Pressure of Oxygen in CdO thin Films. Russ. J. Phys. Chem. B 16, 47–49 (2022). https://doi.org/10.1134/S1990793122010201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122010201