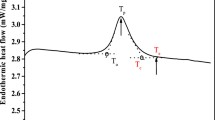
Abstract—The morphology, thermodynamic, and physicochemical properties of maize starch isolated from different maize cultivars with different genotypes are analyzed. The considered maize starches represent four groups of starches: wx, ae, su, and mixed genotypes. All the starches are found to contain granules with oval and irregular shapes, their proportion differing for different genotypes. Starches from the su maize genotype have the smallest granules, compared to the wx and ae starches. An increase in the amylose content of wx and ae maize starches considered here results in the accumulation of defect structures in them, which is seen as a lowering of values for the thermodynamic parameters characterizing their melting. The thermodynamic parameters of dissociation of amylose–lipid complexes (i.e., the temperature and enthalpy) for ae and wx maize starches are inferior to those of the su genotype. All of the considered starch genotypes have similar thicknesses of their crystalline lamellae, practically independent of the amylose content. The dynamic viscosity of gels prepared using the starches decreases as their amylose content increases, irrespective of the genotype of plant they were isolated from.




Similar content being viewed by others
REFERENCES
A. L. Santana and M. A. A. Meireles, Food Publ. Health 4, 229 (2014).
S. C. Zeeman, J. Kossmann, and A. M. Smith, Ann. Rev. Plant Biol. 61, 209 (2010).
A. I. Sergeev, N. G. Shilkina, L. A. Wasserman, S. I. Shilov, and H. Staroszczyk, Russ. J. Phys. Chem. B 11, 361 (2017).
R. M. Aseeva, P. A. Sakharov, and A. M. Sakharov, Russ. J. Phys. Chem. B 3, 844 (2009).
S. Z. Rogovina, K. V. Aleksanyan, L. V. Vladimirov, and A. A. Berlin, Russ. J. Phys. Chem. B 13, 812 (2019).
L. A. Zhorina, O. P. Kuznetsova, S. Z. Rogovina, L. V. Vladimirov, A. V. Grachev, E. V. Prut, and A. A. Berlin, Russ. J. Phys. Chem. B 12, 1076 (2018).
C. M. Durrani and A. M. Donald, Polym. Gels Networks 3, 1 (1995).
J. N. BeMiller and R. L. Whistler, Starch: Chemistry and Technology (Academic, New York, 2009).
C. Christiansen, A. M. Hachem, S. Janecek, A. Vikso-Nielsen, A. Blennow, and B. Svensson, FEBS J. 276, 5006 (2009).
L. A. Wasserman, A. V. Krivandin, A. G. Filatova, V. G. Vasil’ev, O. O. Kolachevskaya, V. F. Tarasov, I. G. Plashchina, and G. A. Romanov, Russ. J. Phys. Chem. B 14, 525 (2020).
J. Liu and A. Mushegian, Protein Sci. 12, 1418 (2003).
M. Leterrier, L. D. Holappa, K. E. Broglie, and D. M. Beckles, BMC Plant Biol. 8, 98 (2008).
M. Shure, S. Wessler, and N. Fedoroff, Cell 35, 225 (1983).
J. Craig, J. R. Lloyd, K. Tomlinson, et al., Plant Cell 10, 13 (1998).
K. Mizuno, K. Kimura, Y. Arai, et al., J. Biochem. 112, 643 (1992).
G. P. Schwall, R. Safford, R. J. Westcott, et al., Nat. Biotechnol. 18, 551 (2000).
A. Nishi, Y. Nakamura, N. Tanaka, and H. Satoh, Plant Physiol. 127, 459 (2001).
C. Sidebottom, M. Kirkland, B. Strongitharm, and R. Jeffcoat, J. Cereal Sci. 27, 279 (1998).
C. Takeda, Y. Takeda, and S. Hizukuri, Carbohyd. Res. 246, 273 (1993).
A. M. Myers, M. K. Morell, M. G. James, and S. G. Ball, Plant Physiol. 122, 989 (2000).
H. Y. Zhang, S. T. Dong, R. Q. Gao, and Y. Q. Li, J. Plant Physiol. Mol. Biol. 33, 25 (2007).
B. Romeis, Mikroskopische Technik (R. Oldenbourg, München, Baltimore, Wien, 1989).
G. K. Adkins and C. T. Greenwood, Starch-Stärke 7, 213 (1966).
C. J. McGrance, H. J. Cornell, and C. J. Rix, Starch 50, 158 (1998).
M. Lille and K. Autio, Inn. Food Sci. Emer. Technol. 8, 117 (2007).
N. R. Andreev, E. N. Kalistratova, L. A. Wasserman, and V. P. Yuryev, Starch 50, 422 (1999).
L. A. Wasserman, A. A. Papakhin, Z. M. Borodina, et al., Carbohydr. Polym. 212, 260 (2019).
P. L. Privalov and S. A. Potekhin, Methods Enzymol. 131, 4 (1986).
Y. I. Matveev, J. J. G. van Soest, C. Nieman, L. A. Wasserman, et al., Carbohyd. Polym. 44, 151 (2001).
A. Imberty, H. Chanzy, S. Perez, A. Buleon, and V. Tran, J. Mol. Biol. 201, 365 (1988).
N. M. Ptitchkina, O. V. Brukhanova, I. A. Novikova, A. G. Ishin, and E. R. Morris, Food Hydrocol. 8, 383 (1994).
S. Nielsen, Food Analysis Laboratory Manual (Springer Int., Switzerland, 2017).
C. G. Biliaderis, Food Technol. 46, 98 (1992).
N. Singh, N. Inouchi, and K. Nishinari, Food Hydrocol. 20, 923 (2006).
P. J. Jenkins and A. M. Donald, Int. J. Biol. Macromol. 17, 315 (1995).
V. A. Bershtein and V. M. Egorov, Differential Scanning Calorimetry of Polymers: Physics, Chemistry, Analysis, Technology, Ed. by T. J. Kemp (Ellis Horwood, New York, London etc., 1994).
V. A. Protserov, L. A. Wasserman, R. F. Tester, et al., Carbohyd. Polym. 49, 271 (2002).
B. Wunderlich, Macromolecular Physics, Vol. 2: Crystal Nucleation, Growth, Annealing (Academic, New York, 1977).
R. F. Tester, Int. J. Biol. Macromol. 21, 37 (1997).
M. A. Whittam, T. R. Noel, and S. G. Ring, Int. J. Biol. Macromol. 12, 359 (1990).
A. Aparicio-Saguilan, G. Méndez-Montealvo, J. Solorza-Feria, and L. A. Bello-Pérez, J. Sci. Food Agric. 86, 1078 (2006).
J. Rosa-Millan, E. Agama-Acevedo, A. R. Jimenez-Aparicio, and L. A. Bello-Pérez, Starch 62, 549 (2010).
Funding
The study was supported as part of state assignments, topics 0084-2014-0005 (no. 01201253307), 0082-2018-0006 (registration no. AAAA-A-18-118020890097-1), nos. 585-2018-0015, and 0662-2019-0006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Kukharuk
Rights and permissions
About this article
Cite this article
Wasserman, L.A., Filatova, A.G., Khatefov, E.B. et al. Some Structural and Thermodynamic Parameters of Maize Starch from Different Maize Genotypes. Russ. J. Phys. Chem. B 15, 161–169 (2021). https://doi.org/10.1134/S1990793121010292
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121010292