Abstract—
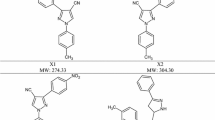
Cyanopyrrolidine derivatives benzyloxycarbonyl-methionyl-cyanopyrrolidine (ZMetPrdN), benzyloxycarbonyl-phenylalanyl-cyanopyrrolidine (ZPhePrdN), tert-butyl-hydroxycarbonyl-glycyl-cyanopyrrolidine (BocGlyPrdN), tert-butyl-hydroxycarbonyl-methionyl-cyanopyrrolidine (BocMetPrdN) are inhibitors of prolyl endopeptidase (PREP; ЕС 3.4.21.26) with the IC50 values ranged from 2 nM to 12 nM. Besides inhibition of PREP, ZMetPrdN, ZPhePrdN, and BocMetPrdN also inhibited activity of dipeptidyl peptidase IV (DPP-4; ЕС 3.4.14.5) with the IC50 values ranged from 1100 nM to 3200 nM. In the acetic acid writhing test in mice all the compounds demonstrated antinociceptive properties. However, only the cyanopyrrolidine derivatives with aromatic substituents (ZMetPrdN and ZPhePrdN) decreased acute exudative inflammation in animals. Three hours after induction of inflammation, the studied cyanopyrrolidine derivatives increased PREP activity and compensatory decreased DPP-4 activity in serum of mice. Thus, one of the components of the mechanism responsible for realization of the antinociceptive and antiexudative properties of the studied derivatives of cyanopyrrolidine is obviously associated with their ability to inhibit PREP activity.

Similar content being viewed by others
REFERENCES
Männistö, P.T. and García-Horsman, J.A., Front. Aging Neurosci., 2017, vol. 9, 27. https://doi.org/10.3389/fnagi.2017.00027
Natunen, T.A., Gynther, M., Rostalski, H., Jaako, K., and Jalkanen, A., Basic Clin. Pharmacol. Toxicol., 2019, vol. 124, no. 1, pp. 40–49. https://doi.org/10.1111/bcpt.13094
O’Reilly, P.J., Hardison, M.T., Jackson, P.L., Xu, X., Snelgrove, R.J., Gaggar, A., Galin, F.S., and Blalock, J.E., J. Neuroimmunol., 2009, vol. 217, nos. 1–2, pp. 51–54. https://doi.org/10.1016/j.jneuroim.2009.09.020
O’Reilly, P., Jackson, P.L., Noerager, B., Parker, S., Dransfield, M., Gaggar, A., and Blalock, J.E. Respir. Res., 2009, vol. 10, 38. https://doi.org/10.1186/1465-9921-10-38
Saidi, M., Kamali, S., and Beaudry, F., Neuropeptides, 2016, vol. 59, pp. 47–55. https://doi.org/10.1016/j.npep.2016.06.002
Skilling, S.R., Smullin, D.H., and Larson, A.A., J. Neurosci., 1990, vol. 10, no. 4, pp. 1309–1318. https://doi.org/10.1523/JNEUROSCI.10-04-01309.1990
Krupina, N.A., Khlebnikova, N.N., Zolotov, N.N., Kushnareva, E.Yu., Bogdanova, N.G., and Orlova, I.N., in Encyclopedia of Pharmacology Research. Series: Pharmacology Research, Safety Testing and Regulation, Cheng, D. and Liu, G., Eds., New York: Nova Science Publishers, 2013, vol. 1, pp. 137–156.
Kalinina, A.P., Kapitsa, I.G., Ivanova, E.A., and Voronina, T.A., Moscow Univ. Biol. Sci. Bull., 2019, vol. 74, no. 2, pp. 69–74. https://doi.org/10.3103/S0096392519020044
Voronina T.A. and Guzevatyh L.S., in Rukovodstvo po provedeniyu doklinicheskikh issledovaniy lekarstvennykh sredstv. Chast’ pervaya. (Guidance on Preclinical Study of New Pharmacological Substances. Part 1), Mironova, A.N. et al., Eds., Moscow: Grif and K, 2012, pp. 197–218.
Shvarts, G.Ya. and Syubaev, R.D., in Rukovodstvo po provedeniyu doklinicheskikh issledovaniy lekarstvennykh sredstv. Chast’ pervaya. (Guidance on Preclinical Study of New Pharmacological Substances. Part 1), Mironova, A.N. et al., Eds., Moscow: Grif and K, 2012, pp. 746–758.
Zolotov, N.N., Kutepova, O.A., Voronina, T.A., Pozdnev, V.F., Smirnov, L.D., and Dyumaev, K.M., Dokl. Akad. Nauk SSSR, 1991, vol. 317, no. 1, pp. 234–237.
Herlihy, S.E., Pilling, D., Maharjan, A.S., and Gomer, R.H., J. Immunol., 2013, vol. 190, no. 12, pp. 6468–6477. https://doi.org/10.4049/jimmunol.1202583
Edgeworth, J., Gorman, M., Bennett, R., Freemont, P., and Hogg, N., J. Biol. Chem., 1991, vol. 266, no. 12, pp. 7706–7713.
Roth, J., Burwinkel, F., van den Bos, C., Goebeler, M., Vollmer, E., and Sorg, C., Blood, 1993, vol. 82, no. 6, pp. 1875–1883.
van den Bos, C., Roth, J., Koch, H.G., Hartmann, M., and Sorg, C., J. Immunol., 1996, vol. 156, no. 3, pp. 1247–1254.
Pagano, R.L., Mariano, M., and Giorgi, R., Mediators Inflamm., 2006, vol. 2006, no. 4, article ID 36765, 1–6. https://doi.org/10.1155/MI/2006/36765
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All manipulations with experimental animals were carried out in accordance with international and Russian regulatory documents: Order of the Ministry of Health of the Russian Federation no. 199n of April 1, 2016 and Directive 2010/63/EU of the European Parliament and the Council of the European Union for the Protection of Animals Used in Scientific purposes. Animals were kept in accordance with the sanitary and epidemiological rules of SR 2.2.1.3218-14 “Sanitary and epidemiological requirements for the design, equipment and maintenance of experimental biological clinics (vivariums).” The experiments were approved by the Commission on Biomedical Ethics of Zakusov Institute of Pharmacology (Protocol no. 1 of January 20, 2017).
CONFLICT OF INTEREST
Authors declare that they have no conflict of interest.
Additional information
Translated by A. Medvedev
Rights and permissions
About this article
Cite this article
Ivanova, E.A., Zolotov, N.N., Pozdnev, V.F. et al. The Effect of Cyanopyrrolidine Derivatives on the Activity of Prolyl Endopeptidase, Acute Exudative Inflammation and Visceral Pain in Mice. Biochem. Moscow Suppl. Ser. B 14, 180–185 (2020). https://doi.org/10.1134/S1990750820020055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750820020055