Abstract
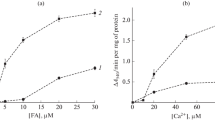
It is known that activated derivatives of long-chain fatty acids acylcarnitines (LCAC) are considered the most toxic, which, along with calcium, can participate in the induction of the mitochondrial pore, involving various types of phospholipases. In this study, the effect of inhibitors of Ca2+-independent and Ca2+-dependent phospholipases, as well as an inhibitor of carnitine palmitoyltransferase on the induction of pores with D,L-palmitoylcarnitine (PC, C16:0) was investigated. In experiments on isolated rat liver mitochondria, the effect of PC on mitochondrial respiration rate, membrane potential (ΔΨm) and mitochondrial swelling during oxidation of glutamate and pyruvate or succinate was studied. It was shown that inhibitors of carnitine palmitoyltransferase-1 etomoxir 2, Ca2+-dependent phospholipase cPLA2 aristolochic acid or Ca2+-independent phospholipase iPLA2γ bromoenol lactone and PACOCF3 caused an increase in critical concentrations of D,L-palmitoylcarnitine (PC*), which were required to decrease the membrane potential and induce mitochondrial swelling. In the ADP activated state 3 (ADP + Mg2+ + hexokinase), Ethomoxir 2 and aristolochic acid promoted the inhibition of respiration and dissipation of membrane potential caused by excess of PC, while phospholipase inhibitors iPLA2γ PACOCF3 and bromoenol lactone provided a pronounced protective effect. Inhibition of iPLA2γ prevented the decrease of ΔΨm and inhibition of respiration caused by PC. Thus, the results obtained indicated the involvement of mitochondrial phospholipase iPLA2γ in the induction of the mitochondrial pore by long-chain acylcarnitines.


Similar content being viewed by others
REFERENCES
Hunter D.R., Haworth R.A. 1979. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 195 (2), 468–477.
Crompton M., Ellinger H., Costi A. 1988. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 255, 357–360.
Bernardi P., Broekemeir K.M., Pfeiffer D.R. 1994. Recent progress on regulation of the mitochondrial permeability transition pore; A cyclosporine-sensitive pore in the inner mitochondrial membrane. J. Bioenerg. Biomembr. 26 (5), 509–517. https://doi.org/10.1007/BF00762735
Kwong J.Q., Molkentin J.D. 2015. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 21 (2), 206. https://doi.org/10.1016/j.cmet.2014.12.001
Ford D.A., Han X., Horner C.C., Gross W. 1996. Accumulation of unsaturated acylcarnitine molecular species during acute myocardial ischemia: Metabolic compartmentalization of products of fatty acyl chain elongation in the acylcarnitine pool. Biochemistry. 35 (24), 7903. https://doi.org/10.1021/bi960552n
Lesnefsky E.J., Moghaddas S., Tandler B., Kerner J., Hoppel C.L. 2001. Mitochondrial dysfunction in cardiac disease: Ischemia–reperfusion, aging, and heart failure. J. Mol. Cell Cardiol. 33 (6), 1065–1089. https://doi.org/10.1006/jmcc.2001.1378
Koves T.R., Ussher J.R., Noland R.C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J.R., Newgard C.B., Lopaschuk G.D., Muoio D.M. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7 (1), 45–56. https://doi.org/10.1016/j.cmet.2007.10.013
Fromenty B., Robin M.A., Igoudjil A., Mansouri A., Pessayre D. 2004. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 30 (2), 121–138. https://doi.org/10.1016/s1262-3636(07)70098-8
Liepinsh E., Makrecka-Kuka M., Volska K., Kuka J., Makarova E., Antone U., Sevostjanovs E., Vilskersts R., Strods A., Tars K., Dambrova M. 2016. Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem. J. 473 (9), 1191–1202. https://doi.org/10.1042/BCJ20160164
Erfle J.D., Sauer F. 1969. The inhibitory effects of acyl-coenzyme A esters on the pyruvate and alpha-oxoglutarate dehydrogenase complexes. Biochim. Biophys. Acta. 178 (3), 441–452. https://doi.org/10.1016/0005-2744(69)90213-7
Lai J.C., Cooper A.J. 1991. Neurotoxicity of ammonia and fatty acids: Differential inhibition of mitochondrial dehydrogenases by ammonia and fatty acyl coenzyme A derivatives. Neurochem. Res. 16 (7), 795–803.
Farrell H.M. Jr, Wickham E.D., Reeves H.C. 1995. Effects of long-chain acyl-coenzyme A’s on the activity of the soluble form of nicotinamide adenine dinucleotide phosphate-specific isocitrate dehydrogenase from lactating bovine mammary gland. Arch. Biochem. Biophys. 321 (1), 199–208. https://doi.org/10.1006/abbi.1995.1386
Paulson D.J., Shug A.L. 1984. Inhibition of the adenine nucleotide translocator by matrix-localized palmityl-CoA in rat heart mitochondria. Biochim. Biophys. Acta. 766 (1), 70–76. https://doi.org/10.1016/0005-2728(84)90218-4.10
Schoënfeld P., Bohnensack R. 1997. Fatty acid-promoted mitochondrial permeability transition by membrane depolarization and binding to the ADP/ATP carrier. FEBS Letters. 420, 167–170.
Ciapaite J., Van Eikenhorst D., Bakker S., Diamant M., Heine R.J., Wagner M.J., Westerhoff H.V., Krab K. 2005. Modular kinetic analysis of the adenine nucleotide translocator-mediated effects of palmitoyl-CoA on the oxidative phosphorylation in isolated rat liver mitochondria. Diabetes. 54 (4), 944–951. https://doi.org/10.2337/diabetes.54.4.944
Wojtczak L., Wieckowski M.R. 1999. The mechanisMC of fatty acid-induced proton permeability of the inner mitochondrial membrane. J. Bioenerg. Biomembr. 31, 447–455.
Sultan A., Sokolove P.M. 2001. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 386 (1), 37–51. https://doi.org/10.1006/abbi.2000.2194
Mironova G. D., Gateau-Roesch O., Levrat C., Gritsenko E., Pavlov E., Lazareva A.V., Limarenko E., Rey C., Louisot P., Saris N.E. 2001. Palmitic and stearic acids bind Ca2+ with high affinity and form nonspecific channels in black-lipid membranes. Possible relation to Ca2+-activated mitochondrial pores. J. Bioenerg. Biomembr. 33 (4), 319–331. https://doi.org/10.1023/a:1010659323937
Mironova G.D., Pavlov E.V. 2021. Mitochondrial cyclosporine A-independent palmitate/Ca2+-induced permeability transition pore (PA-mPT Pore) and its role in mitochondrial function and protection against calcium overload and glutamate toxicity. Cells. 10, 125. https://doi.org/10.3390/cells10010125
Fedotcheva N.I., Grishina E.V., Dynnik V.V. 2022. Induction of mitochondrial cyclosporine-dependent pores by acylcarnitines. Influence of the concentration and length of the carbon chain. Biologicheskie membrany (Rus.). 39 (1), 75–82. https://doi.org/10.31857/S0233475522010066
Dynnik V.V., Grishina E.V., Fedotcheva N.I. 2020. The mitochondrial NO-synthase/guanylate cyclase/protein kinase G signaling system underpins the dual effects of nitric oxide on mitochondrial respiration and opening of the permeability transition pore. FEBS J. 287 (8), 1525–1536. https://doi.org/10.1111/febs.15090
Berezhnov A.V., Fedotova E.I., Nenov M.N., Kasymov V.A., Pimenov O.Y., Dynnik V.V. 2020. Dissecting cellular mechanisMC of long-chain acylcarnitines-driven cardiotoxicity: Disturbance of calcium homeostasis, activation of Ca2+-dependent phospholipases, and mitochondrial energetics collapse. Int. J. Mol. Sci. 21 (20), 7461. https://doi.org/10.3390/ijMC21207461
Hollander J.M., Thapa D., Shepherd D.L. 2014. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am. J. Physiol. Circ. Physiol. 307, H1–H14. https://doi.org/10.1152/ajpheart.00747.2013
Caro A.A., Cederbaum A.I. 2007. Role of intracellular calcium and phospholipase A2 in arachidonic acid-induced toxicity in liver cells overexpressing CYP2E1. Arch. Biochem. Biophys. 457 (2), 252–263. https://doi.org/10.1016/j.abb.2006.10.018
Saito Y., Watanabe K., Fujioka D., Nakamura T., Obata J., Kawabata K., Watanabe Y., Mishina H., Tamaru S., Kita Y., Shimizu T., Kugiyama K. 2012. Disruption of group IVA cytosolic phospholipase A2 attenuates myocardial ischemia-reperfusion injury partly through inhibition of TNF-α-mediated pathway. Am. J. Physiol. Circ. Physiol. 302, H2018–H2030. https://doi.org/10.1152/ajpheart.00955.2011
Asemu G., Dhalla N.S., Tappia P.S. 2004. Inhibition of PLC improves postischemic recovery in isolated rat heart. Am. J. Physiol. Circ. Physiol. 287 (6), H2598–H2605. https://doi.org/10.1152/ajpheart.00506.2004
Hara S., Yoda E., Sasaki Y., Nakatani Y., Kuwata H. 2019. Calcium-independent phospholipase A2γ (iPLA2γ) and its roles in cellular functions and diseases. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 1864 (6), 861–868. https://doi.org/10.1016/j.bbalip.2018.10.009
Moon S.H., Jenkins C.M., Liu X., Guan S., Mancuso D.J., Gross R.W. 2012. Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J. Biol. Chem. 287 (18), 14 880–14 895. https://doi.org/10.1074/jbc.M111.336776
Kinsey G.R., Blum J.L., Covington M.D., Cummings B.S., McHowat J., Schnellmann R. 2008. Decreased iPLA-2gamma expression induces lipid peroxidation and cell death and sensitizes cells to oxidant-induced apoptosis. J. Lipid Res. 49 (7), 1477–1487. https://doi.org/10.1194/jlr.M800030-JLR200
Kinsey G.R., McHowat J., Patrick K.S., Schnellmann R.G. 2007. Role of Ca2+-independent phospholipase A2gamma in Ca2+-induced mitochondrial permeability transition. J. Pharmacol. Exp. Ther. 321 (2), 707–715. https://doi.org/10.1124/jpet.107.119545
Rauckhorst A.J., Broekemeier K.M., Pfeiffer D.R. 2014. Regulation of the Ca2+-independent phospholipase A2 in liver mitochondria by changes in the energetic state. J. Lipid Res. 55 (5), 826–836. https://doi.org/10.1194/jlr.M043307
WilliaMC S.D., Gottlieb R.A. 2002. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem. J. 362 (Pt 1), 23–32. https://doi.org/10.1042/0264-6021:3620023
Moon S.H., Jenkins C.M., Kiebish M.A., SiMC H.F., Mancuso D.J., Gross R.W. 2012. Genetic ablation of calcium-independent phospholipase A(2)γ (iPLA(2)γ) attenuates calcium-induced opening of the mitochondrial permeability transition pore and resultant cytochrome c release. J. Biol. Chem. 287 (35), 29837–29850.https://doi.org/10.1074/jbc.M112.373654
Moon S.H., Liu H., Cedars A.M., Yang C., Kiebish M.A., Joseph S.M., Kelley J., Jenkins C.M., Gross R.W. 2018. Heart failure-induced activation of phospholipase iPLA 2 γ generates hydroxyeicosatetraenoic acids opening the mitochondrial permeability transition pore. J. Biol. Chem. 293 (1), 115–129. https://doi.org/10.1074/jbc.RA117.000405
Moon S.H., Dilthey B.G., Liu X., Guan S., SiMC H.F., Gross R.W. 2021. High-fat diet activates liver iPLA2γ generating eicosanoids that mediate metabolic stress. J. Lipid Res. 62, 100052. https://doi.org/10.1016/j.jlr.2021.100052
Dubinin M.V., Astashev M.E., Penkov N.V., Gudkov S.V., Dyachenko I.A., Samartsev V.N., Belosludtsev K.N. 2016. Effects of phospholipase A2 inhibitors on bilayer lipid membranes. J. Membr. Biol. 249 (3), 339–347. https://doi.org/10.1007/s00232-016-9872-7
Funding
The work was performed under the State Contract 075-00381-21-00 of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences (ITEB RAS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that they have no conflict of interest.
The study was conducted in accordance with the ethical principles formulated in the Helsinki Declaration on the Use of Laboratory Animals and EU Directive 86/609/EEC. All procedures on animals were approved by the Ethics Committee of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences (Protocol 19/2022, March 5, 2022).
Additional information
Translated by E. Puchkov
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fedotcheva, N.I., Grishina, E.V. & Dynnik, V.V. Involvement of the Mitochondrial Ca2+-Independent Phospholipase iPLA2 in the Induction of Mitochondrial Permeability Transition Pore by Long-Chain Acylcarnitines. Biochem. Moscow Suppl. Ser. A 17, 325–331 (2023). https://doi.org/10.1134/S1990747823050045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747823050045